Chemistry:4-Iodopropofol
From HandWiki
Short description: Chemical compound
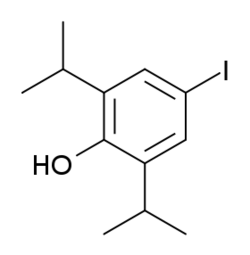 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H17IO |
| Molar mass | 304.171 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
4-Iodopropofol is a drug derived from the commonly used sedative anaesthetic agent, propofol. 4-Iodopropofol has similar effects to propofol on isolated receptors, acting primarily as a GABAA positive modulator and sodium channel blocker,[1][2][3][4] but when given to animals it has only anxiolytic and anticonvulsant effects, lacking the strong sedative-hypnotic profile of propofol.[5]
References
- ↑ "Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors". Journal of Medicinal Chemistry 41 (11): 1846–54. May 1998. doi:10.1021/jm970681h. PMID 9599235.
- ↑ "Anesthetic properties of 4-iodopropofol: implications for mechanisms of anesthesia". Anesthesiology 94 (6): 1050–7. June 2001. doi:10.1097/00000542-200106000-00020. PMID 11465597.
- ↑ "4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABA(A) receptor". Journal of Medicinal Chemistry 45 (15): 3210–21. July 2002. doi:10.1021/jm010461a. PMID 12109905.
- ↑ "High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues". British Journal of Pharmacology 155 (2): 265–75. September 2008. doi:10.1038/bjp.2008.255. PMID 18574460.
- ↑ "Characterization of the electrophysiological and pharmacological effects of 4-iodo-2,6-diisopropylphenol, a propofol analogue devoid of sedative-anaesthetic properties". British Journal of Pharmacology 126 (6): 1444–54. March 1999. doi:10.1038/sj.bjp.0702449. PMID 10217539.
 |

