Biology:Ganaxolone
 | |
| Clinical data | |
|---|---|
| Trade names | Ztalmy |
| Other names | GNX; CCD-1042; 3β-Methyl-5α-pregnan-3α-ol-20-one; 3α-Hydroxy-3β-methyl-5α-pregnan-20-one |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Neurosteroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
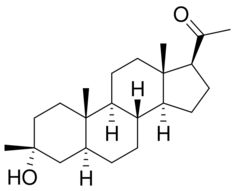
| Formula | C22H36O2 |
| Molar mass | 332.528 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder.[1][3] Ganaxolone is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator.[1]
The most common side effects of treatment with ganaxolone include somnolence (sleepiness), fever, excessive saliva or drooling, and seasonal allergy.[4]
Ganaxolone was approved for medical use in the United States in March 2022,[1][4] and in the European Union in July 2023.[2] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[5][6]
Medical uses
Ganaxolone is indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder.[1][2]
Pharmacology
Mechanism of action
The exact mechanism of action for ganaxolone is unknown; however, results from animal studies suggest that it acts by blocking seizure propagation and elevating seizure thresholds.[7][8]
Ganaxolone is thought to modulate both synaptic and extrasynaptic GABAA receptors to normalize over-excited neurons.[3] Ganaxolone's activation of the extrasynaptic receptor is an additional mechanism that provides stabilizing effects that potentially differentiates it from other drugs that increase GABA signaling.[3]
Ganaxolone binds to allosteric sites of the GABAA receptor to modulate and open the chloride ion channel, resulting in a hyperpolarization of the neuron.[3] This causes an inhibitory effect on neurotransmission, reducing the chance of a successful action potential (depolarization) from occurring.[3][7][8]
It is unknown whether ganaxolone possesses significant hormonal activity in vivo, with a 2020 study finding evidence of in vitro binding to the membrane progesterone receptor.[9]
Chemistry
Ganaxolone is an analog of the neurosteroid allopregnanolone that possesses no known hormonal activity and, instead, is thought to primarily function by binding to GABAA receptors as a positive allosteric modulator.[10]
Other pregnane neurosteroids include alfadolone, alfaxolone, hydroxydione, minaxolone, pregnanolone (eltanolone), and renanolone, among others.[11]
History
The FDA approved ganaxolone based on evidence from a single, double-blind, randomized, placebo-controlled study (Study 1, NCT03572933) of 101 participants with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder who were two years of age and older.[4] The trial was conducted at 36 sites in 8 countries including Australia, France, Israel, Italy, Poland, Russian Federation, the United Kingdom, and the United States.[4] Forty-four (40.7%) of the participants were from US sites.[4] Safety was assessed from a pool of two clinical studies.[4] These include the study of participants with cyclin-dependent kinase-like 5 deficiency disorder and a clinical study that included seven additional participants from a trial of ganaxolone in children and young adults.[4]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 "Ztalmy- ganaxolone suspension". 15 November 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d91612c4-b03a-4be4-a1ee-6a13e3b83d4e.
- ↑ Jump up to: 2.0 2.1 2.2 "Ztalmy EPAR". 31 July 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/ztalmy.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 "Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor". The Journal of Pharmacology and Experimental Therapeutics 280 (3): 1284–1295. March 1997. PMID 9067315.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "Drug Trials Snapshots: Ztalmy". 18 March 2022. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-ztalmy.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". 10 January 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ (PDF) New Drug Therapy Approvals 2022 (Report). January 2024. https://www.fda.gov/media/164429/download. Retrieved 14 January 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jump up to: 7.0 7.1 "Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice". Epilepsia 45 (7): 864–867. July 2004. doi:10.1111/j.0013-9580.2004.04504.x. PMID 15230714.
- ↑ Jump up to: 8.0 8.1 "Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model". Epilepsy Research 89 (2–3): 254–260. May 2010. doi:10.1016/j.eplepsyres.2010.01.009. PMID 20172694.
- ↑ "Anti-apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells". Frontiers in Endocrinology 11 (417): 417. Jun 24, 2020. doi:10.3389/fendo.2020.00417. PMID 32670200.
- ↑ "PubChem compound summary for ganaxolone". National Library of Medicine (National Center for Biotechnology Information). https://pubchem.ncbi.nlm.nih.gov/compound/6918305.
- ↑ , Lorianne K. & Jaakko Lappalainen"Ganaxolone for use in treating genetic epileptic disorders" patent US20190160078A1, issued 2019-05-30
External links
- Clinical trial number NCT03572933 for "Study of Adjunctive Ganaxolone Treatment in Children and Young Adults With CDKL5 Deficiency Disorder (Marigold)" at ClinicalTrials.gov
 |

