Chemistry:N-Desalkylflurazepam
From HandWiki
Short description: Benzodiazepine analog
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
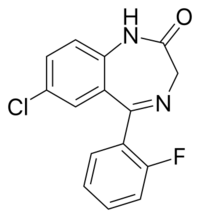
| Formula | C15H10ClFN2O |
| Molar mass | 288.71 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 205 to 206 °C (401 to 403 °F) [1] |
| |
N-Desalkylflurazepam (also known as norflurazepam) is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam,[2] flutoprazepam,[3] fludiazepam,[4] midazolam,[5] flutazolam,[6] quazepam,[7] and ethyl loflazepate.[8][9] It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes.[7] It has been sold as a designer drug from 2016 onward.[10]
References
- ↑ SciFinder record for CAS#2886-65-9
- ↑ "Rapid quantitation of flurazepam and its major metabolite, N-desalkylflurazepam, in human plasma by gas-liquid chromatography with electron-capture detection". Journal of Chromatography 222 (3): 491–5. March 1981. doi:10.1016/S0378-4347(00)84153-5. PMID 7228960.
- ↑ "Pharmacokinetics of flutoprazepam, a novel benzodiazepine drug, in normal subjects". European Journal of Drug Metabolism and Pharmacokinetics 14 (4): 293–8. 1989. doi:10.1007/bf03190114. PMID 2633923.
- ↑ Human Toxicology (1st ed.). Elsevier Science. December 1996. p. 43.
- ↑ "Desalkylflurazepam found in patients' samples after high-dose midazolam treatment". Drug Testing and Analysis 5 (9–10): 745–7. 2013. doi:10.1002/dta.1484. PMID 23713025.
- ↑ "A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry". Forensic Science International 157 (1): 57–70. February 2006. doi:10.1016/j.forsciint.2005.03.011. PMID 15869852.
- ↑ 7.0 7.1 "Comparison of the effects of quazepam and triazolam on cognitive-neuromotor performance". Psychopharmacology 92 (4): 459–64. 1987. doi:10.1007/bf00176478. PMID 2888152.
- ↑ "Pharmacokinetic modeling of ethyl loflazepate (Victan) and its main active metabolites". Annals of Biomedical Engineering 17 (6): 633–46. 1989. doi:10.1007/bf02367467. PMID 2574017.
- ↑ "Determination of circulating ethyl loflazepate metabolites in the baboon by radio-high-performance liquid chromatography with injection of crude plasma samples: comparison with solvent extraction and thin-layer chromatography". Journal of Chromatography 342 (1): 159–65. July 1985. doi:10.1016/S0378-4347(00)84498-9. PMID 2864352.
- ↑ "a and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis 10 (8): 1258–1269. March 2018. doi:10.1002/dta.2387. PMID 29582576. https://rke.abertay.ac.uk/files/14640409/Maskell_ExperimentalVersusTheoreticalLogD7.4_pKa_Accepted_2018.pdf.
 |

