Chemistry:ZK-93423
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
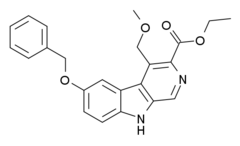
| Formula | C23H22N2O4 |
| Molar mass | 390.439 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
ZK-93423 is an anxiolytic drug from the β-Carboline family, closely related to abecarnil.[1] It is a nonbenzodiazepine GABAA agonist which is not subtype selective and stimulates α1, α2, α3, and α5-subunit containing GABAA receptors equally.[2] It has anticonvulsant, muscle relaxant and appetite stimulating properties comparable to benzodiazepine drugs.[3][4][5][6] ZK-93423 has also been used as a base to develop new and improved beta-carboline derivatives and help map the binding site of the GABAA receptor.[7][8][9][10][11][12]
See also
References
- ↑ "Synthesis of beta- and gamma-carbolines by the palladium-catalyzed iminoannulation of alkynes". The Journal of Organic Chemistry 67 (26): 9318–30. December 2002. doi:10.1021/jo026317u. PMID 12492334.
- ↑ "Discriminative stimulus properties of beta-carbolines characterized as agonists and inverse agonists at central benzodiazepine receptors". Psychopharmacology 83 (3): 233–9. 1984. doi:10.1007/BF00464787. PMID 6089245.
- ↑ "Evaluation of the muscle relaxant properties of a novel beta-carboline, ZK 93423 in rats and cats". British Journal of Pharmacology 86 (2): 357–66. October 1985. doi:10.1111/j.1476-5381.1985.tb08904.x. PMID 3931731.
- ↑ Cooper SJ (January 1986). "Hyperphagic and anorectic effects of beta-carbolines in a palatable food consumption test: comparisons with triazolam and quazepam". European Journal of Pharmacology 120 (3): 257–65. doi:10.1016/0014-2999(86)90466-8. PMID 3753939.
- ↑ "Effects of beta-carbolines in animal models of anxiety". Brain Research Bulletin 19 (3): 293–9. September 1987. doi:10.1016/0361-9230(87)90097-9. PMID 3315125.
- ↑ "Anticonvulsant efficacy of clonazepam and the beta-carboline ZK 93423 during chronic treatment in amygdala-kindled rats". European Journal of Pharmacology 143 (3): 403–14. November 1987. doi:10.1016/0014-2999(87)90464-X. PMID 3691663.
- ↑ "Synthesis of beta-carboline-benzodiazepine hybrid molecules: use of the known structural requirements for benzodiazepine and beta-carboline binding in designing a novel, high-affinity ligand for the benzodiazepine receptor". Journal of Medicinal Chemistry 30 (7): 1248–54. July 1987. doi:10.1021/jm00390a023. PMID 3037081.
- ↑ "Structural requirements for agonist actions at the benzodiazepine receptor: studies with analogues of 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester". Journal of Medicinal Chemistry 33 (3): 1062–9. March 1990. doi:10.1021/jm00165a028. PMID 1968513.
- ↑ "The agonist pharmacophore of the benzodiazepine receptor. Synthesis of a selective anticonvulsant/anxiolytic". Journal of Medicinal Chemistry 34 (5): 1754–6. May 1991. doi:10.1021/jm00109a035. PMID 1674542.
- ↑ "Quantitative structure-activity relationship study of some benzodiazepine-receptor ligands having inverse agonist/antagonist and agonist actions". Drug Design and Discovery 9 (2): 135–43. 1992. PMID 1338366.
- ↑ "Synthesis and evaluation of analogues of the partial agonist 6-(propyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (6-PBC) and the full agonist 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (Zk 93423) at wild type and recombinant GABAA receptors". Journal of Medicinal Chemistry 41 (14): 2537–52. July 1998. doi:10.1021/jm970460b. PMID 9651158.
- ↑ "Structural features controlling the binding of beta-carbolines to the benzodiazepine receptor". Acta Crystallographica B 60 (Pt 4): 481–9. August 2004. doi:10.1107/S0108768104013564. PMID 15258407.
 |

