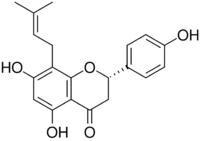
Chemistry:8-Prenylnaringenin
| |
| Names | |
|---|---|
| IUPAC name
(2S)-4′,5,7-Trihydroxy-8-(3-methylbut-2-en-1-yl)flavan-4-one
| |
| Systematic IUPAC name
(2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Hopein; Flavaprenin; Sophoraflavanone B
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H20O5 | |
| Molar mass | 340.375 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
8-Prenylnaringenin (8-PN; also known as flavaprenin, (S)-8-dimethylallylnaringenin, hopein, or sophoraflavanone B) is a prenylflavonoid phytoestrogen. It is reported to be the most estrogenic phytoestrogen known.[1] The compound is equipotent at the two forms of estrogen receptors, ERα and ERβ,[2] and it acts as a full agonist of ERα.[3] Its effects are similar to those of estradiol, but it is considerably less potent in comparison.[2]
8-PN is found in hops (Humulus lupulus) and in beer, and is responsible for the estrogenic effects of the former.[2][4] It can be produced from isoxanthohumol in fungal cells cultures,[5] and by flora in the human intestine.[1][6]
Properties
Estrogenic
8-PN was shown to preserve bone density[1] and has been demonstrated to reduce hot flashes.[1][7] 8-PN also induces the secretion of prolactin, and increases other estrogenic responses.[8] The compound binds to and activates ERα more times[clarification needed] than it does to ERβ.[1][2][9]
This prenylflavanoid has drawn interest in the study of hormone replacement therapy, and it is comparable to some selective estrogen-receptor modulators.[10][11]
In an in vivo study, 8-PN has activated proliferation of mammary cells.[8] At the concentration found in beer, it is unlikely to have an estrogenic effect in breast tissue.[12] Similar to other estrogens, 8-PN induces the expression of the progesterone receptor in various tissues.[8]
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) are suppressed by 8-PN, indicating that it possesses antigonadotropic properties.[8] 8-PN adversely affects male sperm.[13] The role 8-PN plays in fertility requires further research.
Other
In an in vitro study, 8-PN and synthetic derivatives demonstrated anticancer properties.[14] More recently, a radioligand binding study showed enhancements in GABAA receptor activity by 8-PN[15]
Prenylflavonoids from hops, including 8-PN, are ingredients in some breast enlargement supplements,[16] though there is no evidence of its effectiveness for this purpose.[17]
Chemistry
The enzyme naringenin 8-dimethylallyltransferase uses dimethylallyl diphosphate and (−)-(2S)-naringenin to produce diphosphate and sophoraflavanone B (8-prenylnaringenin).
The enzyme 8-dimethylallylnaringenin 2'-hydroxylase uses sophoraflavanone B (8-prenylnaringenin), NADPH, H+ and O2 to produce leachianone G, NADP+ and H2O.
Synthesized derivatives of 8-PN are: 7,4′-di-O-methyl-8-prenylnaringenin; 7-O-pentyl-8-prenylnaringenin; 7,4′-Di-O-allyl-8-prenylnaringenin; 7,4′-Di-O-acetyl-8-prenylnaringenin; and 7,4′-Di-O-palmitoyl-8-prenylnaringenin.[14]
8-Neopentylnaringenin and 8-n-heptylnaringenin are synthetic derivatives of 8-PN.[18]
Etymology
There is another compound, 8-isopentenylnaringenin,[1] also known as sophoraflavanone B, from Sophora flavescens, that could properly be called 8-prenylnaringenin by scientific naming convention.[19]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Hop extracts and hop substances in treatment of menopausal complaints". Planta Med. 79 (7): 576–9. 2013. doi:10.1055/s-0032-1328330. PMID 23512496.
- ↑ 2.0 2.1 2.2 2.3 "Botanical modulation of menopausal symptoms: mechanisms of action?". Planta Med. 79 (7): 538–53. 2013. doi:10.1055/s-0032-1328187. PMID 23408273.
- ↑ Green, Sarah E (2015), In Vitro Comparison of Estrogenic Activities of Popular Women's Health Botanicals, https://indigo.uic.edu/handle/10027/19647, retrieved 2016-01-01
- ↑ Nikolic, D; Li, Y; Chadwick, LR; Grubjesic, S; Schwab, P; Metz, P; Van Breemen, RB (2004). "Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes". Drug Metabolism and Disposition 32 (2): 272–9. doi:10.1124/dmd.32.2.272. PMID 14744951.
- ↑ Fu, Ming-Liang; Wang, Wei; Chen, Feng; Dong, Ya-Chen; Liu, Xiao-jie; Ni, Hui; Chen, Qi-he (2011). "Production of 8-Prenylnaringenin from Isoxanthohumol through Biotransformation by Fungi Cells". Journal of Agricultural and Food Chemistry 59 (13): 7419–26. doi:10.1021/jf2011722. PMID 21634799.
- ↑ Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S. et al. (July 2006). "The Prenylflavonoid Isoxanthohumol from Hops (Humulus lupulus L.) Is Activated into the Potent Phytoestrogen 8-Prenylnaringenin In Vitro and in the Human Intestine". Journal of Nutrition (American Society for Nutrition) 136 (7): 1862–1867. doi:10.1093/jn/136.7.1862. PMID 16772450.
- ↑ Bowe, James (November 15, 2012). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes.". Journal of Endocrinology 191 (2): 399–405. doi:10.1677/joe.1.06919. PMID 17088409.
- ↑ 8.0 8.1 8.2 8.3 Overk, CR; Guo, J; Chadwick, LR; Lantvit, DD; Minassi, A; Appendino, G; Chen, SN; Lankin, DC et al. (2008). "In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin". Chemico-Biological Interactions 176 (1): 30–39. doi:10.1016/j.cbi.2008.06.005. PMID 18619951.
- ↑ Overk, C. R.; Yao, Ping; Chadwick, Lucas R.; Nikolic, Dejan; Sun, Yongkai; Cuendet, Muriel A.; Deng, Yunfan; Hedayat, A. S. et al. (August 2005). "Comparison of the In Vitro Estrogenic Activities of Compounds from Hops (Humulus lupulus) and Red Clover (Trifolium pratense)". J Agric Food Chem 53 (16): 6246–6253. doi:10.1021/jf050448p. PMID 16076101.
- ↑ Rad; Hümpel; Schaefer; Schoemaker; Schleuning; Cohen; Burggraaf (September 1, 2006). "Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women". British Journal of Clinical Pharmacology 62 (3): 288–296. doi:10.1111/j.1365-2125.2006.02656.x. PMID 16934044.
- ↑ Bowe (November 2006). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes.". Journal of Endocrinology 191 (2): 399–405. doi:10.1677/joe.1.06919. PMID 17088409.
- ↑ Bolca, Selin; Li, Jinghu; Nikolic, Dejan; Roche, Nathalie; Blondeel, Phillip; Possemiers, Sam; De Keukeleire, Denis; Bracke, Marc et al. (2010). "Disposition of hop prenylflavonoids in human breast tissue". Molecular Nutrition & Food Research 54 (2): S284–94. doi:10.1002/mnfr.200900519. PMID 20486208.
- ↑ "Environmental 'hormones' wreck sperm". BBC News. July 2, 2002. http://news.bbc.co.uk/2/hi/health/2082449.stm.
- ↑ 14.0 14.1 Anioł, Mirosław (January 7, 2012). "Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4′-O-substituted isoxanthohumols". Med Chem Res 21 (12): 4230–4238. doi:10.1007/s00044-011-9967-8. PMID 23087590.
- ↑ Benkherouf, Ali Y.; Soini, Sanna L.; Stompor, Monika; Uusi-Oukari, Mikko (February 2019). "Positive allosteric modulation of native and recombinant GABAA receptors by hops prenylflavonoids". European Journal of Pharmacology 852: 34–41. doi:10.1016/j.ejphar.2019.02.034. ISSN 0014-2999. PMID 30797788.
- ↑ S. R. Milligan; J. C. Kalita; V. Pocock; V. Van De Kauter; J. F. Stevens; M. L. Deinzer; H. Rong; D. De Keukeleire (December 2000). "The Endocrine Activities of 8-Prenylnaringenin and Related Hop (Humulus lupulus L.) Flavonoids". Journal of Clinical Endocrinology & Metabolism 85 (12): 4912–4915. doi:10.1210/jcem.85.12.7168. PMID 11134162.
- ↑ Chalfoun, Charbel; McDaniel, Candice; Motarjem, Pejman; Evans, Gregory R. D.; Plastic Surgery Educational Foundation DATA Committee (2004). "Breast-Enhancing Pills: Myth and Reality". Plastic and Reconstructive Surgery 114 (5): 1330–3. doi:10.1097/01.PRS.0000141495.14284.8B. PMID 15457059.
- ↑ Breen, L.; Sugden, D.; Heyerick, A.; O'Byrne, K.; Milligan, S. (2009). "The effect of synthetic analogues of the phyto-oestrogen 8-prenylnaringenin on tail skin temperature in a rat hot flush model". Proceedings of the Physiological Society. http://www.physoc.org/proceedings/abstract/Proc%20Physiol%20Soc%2015PC142.
- ↑ Chadwick; Pauli; Farnsworth (July 1, 2005). "The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties". Phytomedicine 13 (1–2): 119–31. doi:10.1016/j.phymed.2004.07.006. PMID 16360942.
 |

