Biology:SC-5233
 | |
| Clinical data | |
|---|---|
| Other names | 6,7-Dihydrocanrenone; 7-Desthioacetylspironolactone; 20-Spirox-4-ene-3,20-dione |
| Routes of administration | By mouth |
| Drug class | Antimineralocorticoid; Progestogen; Steroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H30O3 |
| Molar mass | 342.479 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
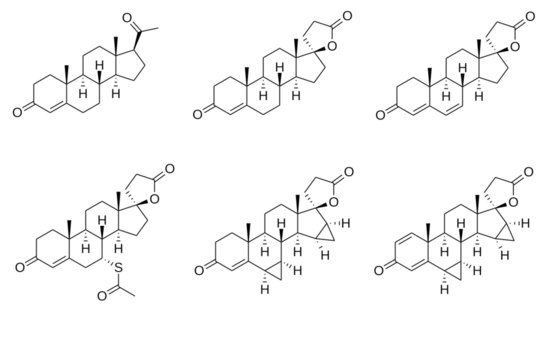
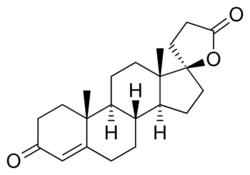
SC-5233, also known as 6,7-dihydrocanrenone or 20-spirox-4-ene-3,20-dione, is a synthetic, steroidal antimineralocorticoid of the spirolactone group which was developed by G. D. Searle & Company in the 1950s but was never marketed.[1][2] It was the first synthetic antagonist of the mineralocorticoid receptor to have been identified and tested in humans.[1][3] The drug was found to lack appreciable oral bioavailability and to be of low potency when administered parenterally,[4] but it nonetheless produced a mild diuretic effect in patients with congestive heart failure.[1] SC-8109, the 19-nor (19-demethyl) analogue, was developed and found to have improved oral bioavailability and potency, but still had low potency.[5] Spironolactone (SC-9420; Aldactone) followed and had both good oral bioavailability and potency, and was the first synthetic antimineralocorticoid to be marketed.[3] It has about 46-fold higher oral potency than SC-5233.[6]
SC-5233 is the propionic acid lactone of testosterone (androst-4-en-17β-ol-3-one) and is also known 3-(3-oxo-17β-hydroxyandrost-4-en-17α-yl)propionic acid γ-lactone or as 17α-(2-carboxyethyl)testosterone γ-lactone.[7] It is the unsubstituted parent or prototype compound of the spirolactone family of steroidal antimineralocorticoids.[2][8]
Similarly to other spirolactones like canrenone and spironolactone, SC-5233 has some antiandrogenic activity and antagonizes the effects of testosterone in animals.[7] In addition, along with SC-8109, it has been found to possess potent progestogenic activity.[9]
Chemical structures of spirolactones
|
References
- ↑ 1.0 1.1 1.2 "Diuretics in Liver Disease". Diuresis and Diuretics / Diurese und Diuretica: An International Symposium Herrenchiemsee, June 17th–20th, 1959 Sponsored by CIBA / Ein Internationales Symposium Herrenchiemsee, 17.–20. Juni 1959 Veranstaltet mit Unterstützung der CIBA. Springer-Verlag. 14 December 2013. pp. 224, 261. doi:10.1007/978-3-642-92756-0_11. ISBN 978-3-642-49716-2. https://books.google.com/books?id=BJKhBgAAQBAJ&pg=PA261.
- ↑ 2.0 2.1 "Diuretics". Pharmaceutical Chemistry of Antihypertensive Agents. CRC Press. 19 December 1990. pp. 82–. ISBN 978-0-8493-4724-5. https://books.google.com/books?id=LMl4Sd38q4kC&pg=PA82%7CDATE%3D19+DECEMBER+1990%7CPUBLISHER%3DCRC+PRESS%7CISBN%3D978-0-8493-4724-5%7CPAGES%3D82%E2%80%9383%7D%7D%3C%2FREF%3E.
- ↑ 3.0 3.1 "The Renin-Angiotensin-Aldosterone System in Cardiovascular Disease". Introduction to Translational Cardiovascular Research. Springer. 6 November 2014. pp. 61–. ISBN 978-3-319-08798-6. https://books.google.com/books?id=DP05BQAAQBAJ&pg=PA61.
- ↑ "Spironolactone". The British Encyclopaedia of Medical Practice: Medical progress. Butterworth & Company. 1961. p. 302. https://books.google.com/books?id=wvIfAQAAMAAJ. "Cena and Kagawa first synthesized 3-(3-oxo-17β-hydroxy-4-androsten-17α-yl)-propionic acid-gamma-lactone and later prepared its 19-nor analogue. These compounds were designated SC-5233 and SC-8109, respectively. Both have anti-aldosterone activity and most of the early work on aldosterone antagonism was done with their aid. SC-5233 is not appreciably absorbed when given by mouth and the parenteral dose is large. As in the case of certain other steroids the 19-nor derivative was an improvement on the parent compound (Klyne, 1959). SC-8109 is well absorbed from the alimentary tract, but the dose is about 2 g daily."
- ↑ Corticosteroids in medical practice. Thomas. 1 January 1962. p. 310. ISBN 9780398002152. https://books.google.com/books?id=UtVqAAAAMAAJ.
- ↑ "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". The Journal of Endocrinology 234 (1): T125–T140. July 2017. doi:10.1530/JOE-16-0600. PMID 28634268.
- ↑ 7.0 7.1 "Pharmacology of a new steroid that blocks salt activity of aldosterone and desoxycorticosterone". The Journal of Pharmacology and Experimental Therapeutics 126 (2): 123–130. June 1959. PMID 13665517. "[SC-5233] (total dose of 5 mg/rat) partially blocked the effects of testosterone propionate on the seminal vesicles and prostate in similar animals.".
- ↑ "Eplerenone: Selective Aldosterone Antagonist". Analogue-based Drug Discovery II. John Wiley & Sons. 24 August 2010. pp. 361–. ISBN 978-3-527-63212-1. https://books.google.com/books?id=h2Kd8ci4Ln8C&pg=PA361.
- ↑ "Progestational activity of certain steroid-17-spirolactones". Proceedings of the Society for Experimental Biology and Medicine 99 (2): 451–452. November 1958. doi:10.3181/00379727-99-24380. PMID 13601900.
 |