Chemistry:Fosfestrol
 | |
| Clinical data | |
|---|---|
| Trade names | Honvan, others |
| Other names | Diethylstilbestrol diphosphate; Stilbestrol diphosphate; DESDP; DESP; DES-DP; DES-P |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous, by mouth |
| Drug class | Nonsteroidal estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H22O8P2 |
| Molar mass | 428.314 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fosfestrol, sold under the brand name Honvan and also known as diethylstilbestrol diphosphate (DESDP), is an estrogen medication which is used in the treatment of prostate cancer in men.[1][2][3] It is given by slow intravenous infusion once per day to once per week or by mouth once per day.[3][2]
Side effects of fosfestrol include nausea and vomiting, cardiovascular complications, blood clots, edema, and genital skin reactions, among others.[2] Fosfestrol is an estrogen, and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[2][1][4] It acts as a prodrug of diethylstilbestrol.[2][1][5]
Fosfestrol was patented in 1941 and was introduced for medical use in 1955.[6] It was previously marketed widely throughout the world, but now remains available in only a few countries.[7][8][6][3]
Medical uses
Fosfestrol is used as a form of high-dose estrogen therapy in the treatment of castration-resistant prostate cancer.[2] It is added once progression of metastases has occurred following therapy with other interventions such orchiectomy, gonadotropin-releasing hormone modulators, and nonsteroidal antiandrogens.[2] Fosfestrol has also been used to prevent the testosterone flare at the start of gonadotropin-releasing hormone agonist therapy in men with prostate cancer.[9]
Fosfestrol sodium is given at a dosage of 600 to 1200 mg/day by slow intravenous infusion over a period of 1 hour for a treatment duration of 5 to 10 days in men with prostate cancer.[3][2] Following this, it is given at a dose of 300 mg/day for 10 to 20 days.[3] Maintenance doses of fosfestrol sodium of 300 to 600 mg may be given four times per week.[3] This may be gradually reduced to one 300 to 600-mg dose per week over a period of several months.[3]
Fosfestrol sodium is also used to a lesser extent by oral administration initially at a dosage of 360 to 480 mg three times per day in the treatment of prostate cancer.[3][2] Maintenance doses of 120 to 240 mg three times per day may be used and can be gradually reduced to 240 mg/day.[3][2]
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Available forms
Fosfestrol is available in the form of solutions for intravenous administration and tablets for oral administration.[10]
Side effects
Side effects of fosfestrol include nausea and vomiting in 80% of patients (with 1 in 25 cases, or 4%, resulting in death), cardiovascular complications (18% with fosfestrol plus adriamycin relative to 2% with adriamycin alone) such as thrombosis (2 in 25 cases, or 8%), edema (44% requiring diuretic therapy), and skin reactions such as burning, itching, or pain in the genital area (40%).[2][1] In addition, weight gain, feminization, and gynecomastia may occur.[1]
Pharmacology
Pharmacodynamics

Fosfestrol is an estrogen, or an agonist of the estrogen receptors.[2][1][4] It is inactive itself and acts as a prodrug of diethylstilbestrol.[2][1][5] Similarly to diethylstilbestrol, fosfestrol has powerful antigonadotropic effects and strongly suppresses testosterone levels in men.[2][1][12][13] It decreases testosterone levels into the castrate range within 12 hours of the initiation of therapy.[1] Fosfestrol may also act by other mechanisms, such as via direct cytotoxic effects in the prostate gland.[2][1]
Pharmacokinetics
The pharmacokinetics of fosfestrol have been studied.[2][14][1]
Chemistry
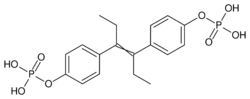
Fosfestrol is a synthetic nonsteroidal estrogen of the stilbestrol group.[15][3] It is an estrogen ester; specifically, it is the diphosphate ester of diethylstilbestrol.[15][3]
Fosfestrol is provided both as the free base and as a tetrasodium salt.[2][3] In terms of dose equivalence, 300 mg anhydrous fosfestrol sodium is equal to about 250 mg fosfestrol.[3]
A polymer of fosfestrol, polydiethylstilbestrol phosphate, was developed as a long-acting estrogen for potential use in veterinary medicine, but was never marketed.[16][17][18][19][20][21]
History
Fosfestrol was first patented in 1941 and was mentioned in the literature by Huggins.[6][22] Conjugated estrogens and diethylstilbestrol sulfate, which are water-soluble estrogens, were first reported to be effective in the treatment of prostate cancer via intravenous administration in 1952.[23][22] Starting in October 1952, Flocks and colleagues studied intravenous fosfestrol in the treatment of prostate cancer, publishing their findings in 1955.[22] Fosfestrol was first introduced for medical use in 1955 under the brand names Stilphostrol and ST 52 in the United States and France , respectively.[6]
Society and culture
Generic names
Fosfestrol is the generic name of the drug and its INN, BAN, and JAN, while diethylstilbestrol diphosphate is its USAN and fosfestrolo is its DCIT.[15][7][8][3] It is also known as stilbestrol diphosphate.[15][7][8] Fosfestrol sodium is its INNM and BANM.[15][7][8][3]
Brand names
Brand names of fosfestrol include Cytonal, Difostilben, Honovan, Honvan, Honvol, Honvon, Fosfostilben, Fostrolin, ST 52, Stilbetin, Stilbol, Stilbostatin, Stilphostrol, and Vagestrol, among others.[15][7][8][6]
Availability
Fosfestrol has been marketed widely throughout the world, including in the United States , Canada , Europe, Asia, Latin America, and South Africa , among other areas of the world.[7][8][3][6] However, today, it appears to remain available only in a few countries, including Bangladesh, Egypt, India , Oman, and Tunisia.[8][3]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "High-dose continuous-infusion fosfestrol in hormone-resistant prostate cancer". Cancer 71 (3 Suppl): 1123–1130. February 1993. doi:10.1002/1097-0142(19930201)71:3+<1123::AID-CNCR2820711434>3.0.CO;2-T. PMID 8428334.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 Hagers Handbuch der Pharmazeutischen Praxis: Band 8: Stoffe E-O. Springer-Verlag. 2 July 2013. pp. 301–. ISBN 978-3-642-57994-3. https://books.google.com/books?id=8vSjBgAAQBAJ&pg=PA301.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. 2009. pp. 2104–2105. ISBN 978-0-85369-840-1. https://www.medicinescomplete.com/mc/martindale/.
- ↑ 4.0 4.1 "Estrogens and Antiestrogens in the Male". Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Handbook of Experimental Pharmacology. 135 / 2. Springer Science & Business Media. 1999. pp. 505–571. doi:10.1007/978-3-642-60107-1_25. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA538.
- ↑ 5.0 5.1 Urotext (1 January 2001). Urotext-Luts: Urology. Urotext. pp. 386–. ISBN 978-1-903737-03-3. https://books.google.com/books?id=6zjtA37qDsMC&pg=PA386.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1292–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1292.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 332–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA332.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 "Fosfestrol - Drugs.com". https://www.drugs.com/international/fosfestrol.html.
- ↑ "Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: a multicenter, randomized, controlled trial in Japan. Zoladex Study Group". Japanese Journal of Clinical Oncology 29 (11): 562–570. November 1999. doi:10.1093/jjco/29.11.562. PMID 10678560.
- ↑ Modell's Drugs in Current Use and New Drugs, 2006: 52nd Edition. Springer Publishing Company. 8 February 2006. pp. 206–. ISBN 978-0-8261-7097-2. https://books.google.com/books?id=-y5sb6ueXrUC&pg=PA206.
- ↑ 11.0 11.1 11.2 "Plasma testosterone: an accurate monitor of hormone treatment in prostatic cancer". British Journal of Urology 45 (6): 668–677. December 1973. doi:10.1111/j.1464-410x.1973.tb12238.x. PMID 4359746.
- ↑ "Effects of intravenous administration of high dose-diethylstilbestrol diphosphate on serum hormonal levels in patients with hormone-refractory prostate cancer". Endocrine Journal 46 (5): 659–664. October 1999. doi:10.1507/endocrj.46.659. PMID 10670751.
- ↑ "Effekt von Diäthylstilböstroldiphosphat auf die Serumkonzentration von Testosteron und Luteinisierungshormon beim M1-Prostatakarzinom". Verhandlungsbericht der Deutschen Gesellschaft für Urologie. 32. 1981. pp. 447–449. doi:10.1007/978-3-642-81706-9_133. ISBN 978-3-540-11017-0.
- ↑ "New Results on the Pharmacokinetics of Fosfestrol". Urologia Internationalis 43 (1): 15–23. 1988. doi:10.1159/000281427. ISSN 1423-0399.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 396–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA396.
- ↑ "High Molecular Weight Enzyme Inhibitors. IV. Polymeric Phosphates of Synthetic Estrogens.". Acta Chem. Scand. 13 (5): 1011–1018. 1959. doi:10.3891/acta.chem.scand.13-1011. http://actachemscand.org/pdf/acta_vol_13_p1011-1018.pdf.
- ↑ "Autoradiographic Distribution Studies after Administration of a Macromolecular Synthetic Oestrogen (14C-Polydiethylstilboestrol Phosphate)". Acta Endocrinologica 43 (4): 571–580. August 1963. doi:10.1530/acta.0.0430571. PMID 14059878.
- ↑ "Distribution and Excretion of Radioactivity after Parenteral Administration of Radioactive Polydiethylstilbestrol Phosphate to Rats and a Cow". Proceedings of the Society for Experimental Biology and Medicine 117 (2): 394–398. November 1964. doi:10.3181/00379727-117-29590. PMID 14233451.
- ↑ "Excretion of radioactivity by human subjects after ingestion of liver from cattle treated with labeled polydiethylstilbestrol phosphate". Proceedings of the Society for Experimental Biology and Medicine 119 (4): 996–998. 1965. doi:10.3181/00379727-119-30359. PMID 5891085.
- ↑ "Distribution and excretion of 14C-labeled polydiethylstilbestrol phosphate in a steer". Journal of Animal Science 26 (5): 1094–1100. September 1967. doi:10.2527/jas1967.2651094x. PMID 6077168.
- ↑ "The veterinarian and intensive livestock production: humane considerations". The Canadian Veterinary Journal 13 (10): 229–233. October 1972. PMID 4562986.
- ↑ 22.0 22.1 22.2 "Prostatic carcinoma: treatment of advanced cases with intravenous diethylstilbestrol diphosphate". The Journal of Urology 74 (4): 549–551. October 1955. doi:10.1016/S0022-5347(17)67313-0. PMID 13264317.
- ↑ Advanced Therapy of Prostate Disease. PMPH-USA. 2000. pp. 381–. ISBN 978-1-55009-102-1. https://books.google.com/books?id=9AKuf7rzfjcC&pg=PA381.
Further reading
- "Chemotherapy of prostatic cancer". The Urologic Clinics of North America 2 (1): 185–196. February 1975. doi:10.1016/S0094-0143(21)01066-1. PMID 1093059.
- "High-dose continuous-infusion fosfestrol in hormone-resistant prostate cancer". Cancer 71 (3 Suppl): 1123–1130. February 1993. doi:10.1002/1097-0142(19930201)71:3+<1123::AID-CNCR2820711434>3.0.CO;2-T. PMID 8428334.
- "[High-dose intravenous diethylstilbestrol diphosphate therapy for hormone refractory prostate cancer]" (in ja). Nihon Rinsho. Japanese Journal of Clinical Medicine 56 (8): 2145–2149. August 1998. PMID 9750524.
- "[Estrogen therapy--high-dose intravenous diethylstilbestrol diphosphate therapy for advanced or hormone refractory prostate cancer]" (in ja). Nihon Rinsho. Japanese Journal of Clinical Medicine 60 (Suppl 11): 199–204. December 2002. PMID 12599571.
 |

