Chemistry:Pharmacological treatment of Parkinson's disease
An antiparkinson medication is a type of drug which is intended to treat and relieve the symptoms of Parkinson's disease. Most of these agents act by either increasing dopamine activity or reducing acetylcholine activity in the central nervous system.
Parkinson's disease
Parkinson's disease is a motor system disorder of the nervous system. It is outlined as a progressive disorder that affects movement and results in the loss of dopamine-producing brain cells, causing tremor in the hands, arms, legs, jaw, and face and/or rigidity or stiffness of the limbs and trunk.[1] The primary symptoms are muscular rigidity, slowness of movement, a resting tremor, and postural instability.[2] Parkinson's disease is caused by degeneration of the nigrostriatal system, which is the dopamine-secreting neurons of the substantia nigra that send axons to the basal ganglia.[2] The basal ganglia control the automatic, habitual responses performed by the human body.
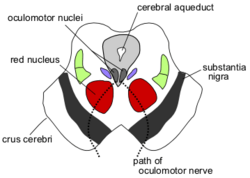
Diagnosing Parkinson's disease is difficult, as no specific test exists for it. Doctors usually perform other tests to rule out other conditions.[3] Lewy bodies are often seen in the dopaminergic neurons in the brains of patients who have Parkinson's disease; they are abnormal circular structures found within the cytoplasm. Lewy bodies have a dense protein core, surrounded by a halo of radiating fibers.[2] Mutations on chromosome 4 can cause Parkinson's disease. This gene produces a protein known as a-synuclein, which is normally found in the presynaptic terminals and is thought to be involved in synaptic transmission in dopaminergic neurons. The mutation produces what is known as a toxic gain of function because it produces a protein that results in effects that are toxic to the cell. Parkinson's disease can also be caused by a mutation on chromosome 6. This gene has been named parkin; its mutation causes a loss of function, and it is a recessive disorder.[2]
Common medication
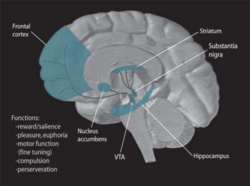
The goal of the most common antiparkinson drugs is to either replace the dopamine levels in the brain or mimic the actions of dopamine. The main categories of antiparkinson drugs are anticholinergic drugs and dopaminergic drugs. Anticholinergic drugs block the action of acetylcholine, compensating for the low levels of dopamine. As stated before, dopaminergic drugs aim to replace dopamine or inhibit the degradation of dopamine in the brain.[4]
L-DOPA
Once a preliminary diagnosis is made, carbidopa-levodopa can be given as an antiparkinson medication. If this medication shows improvement, doctors likely can confirm their diagnoses. This standard treatment for Parkinson's disease is referred to as L-DOPA, the precursor of dopamine. L-DOPA causes the person's remaining dopaminergic neurons to produce and secrete more dopamine, thus counteracting the effects of Parkinson's disease. However, the nigrostriatal dopaminergic neurons in the brain eventually drop to a low enough count to where the symptoms of Parkinson's disease become worse. This is due to the short half-life of L-DOPA in the body, typically 1.5–2.0 hours.[5] L-DOPA also activates DA neurons in the mesolimbic/mesocortical system and produces side effects such as hallucinations and delusions.[2]
Deprenyl
A medicine that can be given with L-DOPA, or separately, is deprenyl. It inhibits the activity of the enzyme MAO-B, which then will slow the progression of Parkinson's disease. Deprenyl, however, does not completely stop the degeneration of dopaminergic neurons.[2] Deprenyl delays the time before other antiparkinson drugs, like L-DOPA, need to be used.[6]
Tyrosine hydroxylase
Tyrosine hydroxylase catalyzes the formation of L-DOPA, the rate-limiting step in the biosynthesis of dopamine.[7] In other words, it is a precursor to neurotransmitters and increases plasma neurotransmitter levels of dopamine and norepinephrine.[8] This medication should not be used when taking L-DOPA, as L-DOPA interferes with the absorption of Tyrosine.[9]
Apomorphine
Apomorphine has also been used to treat Parkinson's disease. It is a dopamine receptor agonist, but it does cause severe side effects when used on its own.[10]
Anticholinergic drugs
Anticholinergic drugs include benzhexol and orphenadrine, which reduce the effect of acetylcholine in the brain by antagonizing cholinergic receptors. This helps restore the acetylcholine/dopamine balance within the brain. Again with these treatments, about 70% of patients taking anticholinergics develop serious side effects, including hallucinations, dyskinetic movements, vision effects, difficulty swallowing, dry mouth, and urine retention.[10]
mGluR4
N-phenyl-7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) is now being studied as a selective allosteric potentiator of mGluR4. Metabotropic glutamate receptor 4 (mGluR4) is a potential drug target for Parkinson's disease. PHCCC selectively potentiated agonist-induced mGluR4 activity in cells expressing this receptor and did not itself act as an agonist. PHCCC also potentiated the effect of L-(+)-2-amino-4-phosphonobutyric acid in inhibiting transmission at the striatopallidal synapse. This is significant due to the striatopallidal synapse being proposed as a target for Parkinson's disease treatment. This can hopefully restore balance in the basal ganglial motor circuit.[11]
Research
Recent research for alleviating the symptoms of Parkinson's disease involves stereotaxic procedures, which are typically done when patients no longer respond to antiparkinson drugs. These procedures include transplantation of fetal tissue in an attempt to re-establish the secretion of dopamine in the neostriatum. Researchers have also attempted strategies of gene therapy. A genetically modified virus was inserted into the subthalamic nucleus of patients with Parkinson's disease. This virus delivered a gene for glutamate decarboxylase (GAD), which is the enzyme responsible for the biosynthesis of the major inhibitory neurotransmitter, GABA. In the study, GAD turned some of the excitatory glutamate-producing neurons in the subthalamic nucleus into inhibitory, GABA-producing neurons, improving the symptoms of Parkinson's disease.[2]
Examples
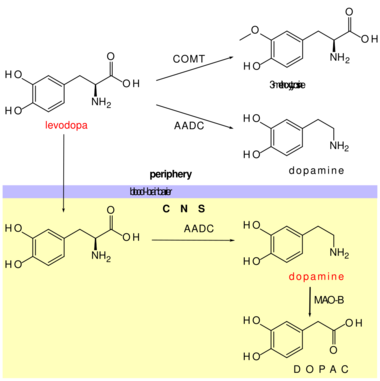
- Dopaminergic precursors are preferred over other medications to prevent undesirable sympathomimetic side effects.
- COMT inhibitors prevent the peripheral metabolism of levodopa by COMT, hence increase its brain levels.
- Entacapone
- Opicapone
- Tolcapone (also acts in the CNS)[13])
- Peripheral aromatic L-amino acid decarboxylase inhibitors (DOPA decarboxylase inhibitors) prevent the peripheral metabolism of levodopa by decarboxylases, hence increase its brain levels.
- Selective monoamine oxidase B inhibitors prevent the metabolism of dopamine by MAOB, hence increase its brain levels.
- Dopamine receptor agonists directly increase the activity of the dopamine system.
- Anticholinergics
- Antimuscarinics
- Benzatropine
- Diphenhydramine
- Dimenhydrinate
- Scopolamine
References
- ↑ NINDS. "NINDS Parkinson's Disease Information Page." Parkinson's Disease Information Page: National Institute of Neurological Disorders and Stroke (NINDS). National Institute of Neurological Disorders and Stroke, 12 Mar. 2014. Web. 12 Mar. 2014.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Neil Carlson. Pearson Education, Inc.. ed. Psychology of Behavior (11 ed.). pp. 533–538.
- ↑ Mayo Clinic Staff. "Parkinson's Disease." Diagnosis at Mayo Clinic. Mayo Foundation for Medical Education and Research, 2014. Web. 12 Mar. 2014.
- ↑ Parkinson's UK. "Anticholinergics". http://www.parkinsons.org.uk/content/anticholinergics. Retrieved 30 April 2014.
- ↑ NIH DailyMed. "Sinemet". http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=be8e535a-2f3a-42b1-bb18-ab88b20eea99. Retrieved 21 August 2014.
- ↑ Tetrud, JW; JW Langston (4 August 1989). "The effect of deprenyl (selegiline) on the natural history of Parkinson's disease". Science 245 (4917): 519–522. doi:10.1126/science.2502843. PMID 2502843. Bibcode: 1989Sci...245..519T.
- ↑ Haavik, J; Toska, K. (June 1998). "Tyrosine Hydroxylase and Parkinson's". Mol. Neurobiol. 16 (3): 285–309. doi:10.1007/BF02741387. PMID 9626667.
- ↑ Rasmussen, DD; Ishizuka, B; Quigley, ME; Yen, SS (1983). "Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations". J. Clin. Endocrinol. Metab. 57 (4): 760–3. doi:10.1210/jcem-57-4-760. PMID 6885965.
- ↑ University of Maryland Medical Center. "Tyrosine". https://umm.edu/health/medical/altmed/supplement/tyrosine. Retrieved 30 April 2014.
- ↑ Jump up to: 10.0 10.1 Donovan, Stephen. "Parkinson's Disease Treatment". http://www.google.com/patents/US6620415. Retrieved 16 April 2014.
- ↑ Marino, Michael (11 November 2003). "Allosteric modulation of group III metabotropic glutamate receptor 4: A potential approach to Parkinson's disease treatment". PNAS 100 (23): 13668–13673. doi:10.1073/pnas.1835724100. PMID 14593202. Bibcode: 2003PNAS..10013668M.
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001) (in German). Arzneimittelwirkungen (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 313–316. ISBN 3-8047-1763-2.
- ↑ (in German) Arzneistoff-Profile. 10 (13 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. 1998. ISBN 978-3-7741-9846-3.

