Chemistry:Tropoflavin
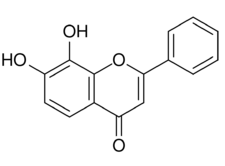 | |
| Clinical data | |
|---|---|
| Other names | 7,8-Dihydroxyflavone |
| Pharmacokinetic data | |
| Bioavailability | ~5% (in mice)[1] |
| Elimination half-life | < 30 minutes (in mice)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C15H10O4 |
| Molar mass | 254.241 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tropoflavin, also known as 7,8-dihydroxyflavone, is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves.[2][3][4] It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB) (Kd ≈ 320 nM), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF).[5][6][7] Tropoflavin is both orally bioavailable and able to penetrate the blood–brain barrier.[8][9] A prodrug of tropoflavin with greatly improved potency and pharmacokinetics, R13 (and, formerly, R7), is under development for the treatment of Alzheimer's disease.[10][11]
Tropoflavin has demonstrated therapeutic efficacy in animal models of a variety of central nervous system disorders,[7] including depression,[8] Alzheimer's disease,[12][13][14] cognitive deficits in schizophrenia,[15] Parkinson's disease,[5] Huntington's disease,[16] amyotrophic lateral sclerosis,[17] traumatic brain injury,[18] cerebral ischemia,[19][20] fragile X syndrome,[21] and Rett syndrome.[22] Tropoflavin also shows efficacy in animal models of age-associated cognitive impairment[23] and enhances memory consolidation and emotional learning in healthy rodents.[24][25] In addition, tropoflavin possesses powerful antioxidant activity independent of its actions on the TrkB receptor,[26] and protects against glutamate-induced excitotoxicity,[27] 6-hydroxydopamine-induced dopaminergic neurotoxicity,[28] and oxidative stress-induced genotoxicity.[29] It was also found to block methamphetamine-induced dopaminergic neurotoxicity, an effect which, in contrast to the preceding, was found to be TrkB-dependent.[30]
In 2017, evidence was published suggesting that tropoflavin and various other reported small-molecule TrkB agonists might not actually be direct agonists of the TrkB and might be mediating their observed effects by other means.[31][32]
Tropoflavin has been found to act as a weak aromatase inhibitor in vitro (Ki = 10 μM),[33] though there is evidence to suggest that this might not be the case in vivo.[5] In addition, it has been found to inhibit aldehyde dehydrogenase and estrogen sulfotransferase in vitro (Ki = 35 μM and 1–3 μM, respectively), though similarly to the case of aromatase, these activities have not yet been confirmed in vivo.[5] Unlike many other flavonoids, tropoflavin does not show any inhibitory activity on 17β-hydroxysteroid dehydrogenase.[34] Tropoflavin has also been observed to possess in vitro antiestrogenic effects at very high concentrations (Ki = 50 μM).[35][36]
A variety of close structural analogues of tropoflavin have also been found to act as TrkB agonists in vitro, including diosmetin (5,7,3'-trihydroxy-4'-methoxyflavone), norwogonin (5,7,8-trihydroxyflavone), eutropoflavin (4'-dimethylamino-7,8-dihydroxyflavone), 7,8,3'-trihydroxyflavone, 7,3'-dihydroxyflavone, 7,8,2'-trihydroxyflavone, 3,7,8,2'-tetrahydroxyflavone, and 3,7-dihydroxyflavone.[37] The highly hydroxylated analogue gossypetin (3,5,7,8,3',4'-hexahydroxyflavone), conversely, appears to be an antagonist of TrkB in vitro.[37]
Tropoflavin was also found to decrease mouse sleep in dark phase and reduce hypothalamus level of orexin A but not orexin B in mice.[38]
See also
References
- ↑ 1.0 1.1 "7,8-Dihydoxyflavone and 7,8-substituted flavone derivatives, compositions, and methods related thereto" US patent application 20150274692, published 2015-10-01, assigned to Emory University
- ↑ "Fear extinction and BDNF: translating animal models of PTSD to the clinic". Genes, Brain and Behavior 11 (5): 503–12. July 2012. doi:10.1111/j.1601-183X.2012.00801.x. PMID 22530815.
- ↑ "Farinose alpine Primula species: phytochemical and morphological investigations". Phytochemistry 98: 151–9. February 2014. doi:10.1016/j.phytochem.2013.11.018. PMID 24345641. Bibcode: 2014PChem..98..151C.
- ↑ Cell Press (2015). "Molecule found in tree leaves helps female mice combat weight gain; males unaffected". ScienceDaily. https://www.sciencedaily.com/releases/2015/03/150305125355.htm.
- ↑ 5.0 5.1 5.2 5.3 "A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone". Proc. Natl. Acad. Sci. U.S.A. 107 (6): 2687–92. 2010. doi:10.1073/pnas.0913572107. PMID 20133810. Bibcode: 2010PNAS..107.2687J.
- ↑ "Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor". J. Biol. Chem. 289 (40): 27571–84. 2014. doi:10.1074/jbc.M114.562561. PMID 25143381.
- ↑ 7.0 7.1 "Small molecules activating TrkB receptor for treating a variety of CNS disorders". CNS Neurol Disord Drug Targets 12 (7): 1066–77. 2013. doi:10.2174/18715273113129990089. PMID 23844685.
- ↑ 8.0 8.1 "A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect". J. Med. Chem. 53 (23): 8274–86. 2010. doi:10.1021/jm101206p. PMID 21073191.
- ↑ "Optimization of a small tropomyosin-related kinase B (TrkB) agonist 7,8-dihydroxyflavone active in mouse models of depression". J. Med. Chem. 55 (19): 8524–37. 2012. doi:10.1021/jm301099x. PMID 22984948.
- ↑ "The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer's disease". Proc. Natl. Acad. Sci. U.S.A. 115 (3): 578–583. January 2018. doi:10.1073/pnas.1718683115. PMID 29295929. Bibcode: 2018PNAS..115..578C.
- ↑ "7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders". Translational Neurodegeneration 5 (1): 2. 2016. doi:10.1186/s40035-015-0048-7. PMID 26740873.
- ↑ "7,8-Dihydroxyflavone, a small molecule TrkB agonist, improves spatial memory and increases thin spine density in a mouse model of Alzheimer disease-like neuronal loss". PLOS ONE 9 (3): e91453. 2014. doi:10.1371/journal.pone.0091453. PMID 24614170. Bibcode: 2014PLoSO...991453C.
- ↑ "7,8-dihydroxyflavone ameliorates scopolamine-induced Alzheimer-like pathologic dysfunction". Rejuvenation Res 17 (3): 249–54. 2014. doi:10.1089/rej.2013.1519. PMID 24325271.
- ↑ "7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer's disease". Neuropsychopharmacology 39 (3): 638–50. 2014. doi:10.1038/npp.2013.243. PMID 24022672.
- ↑ "Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia". Pharmacol. Biochem. Behav. 122: 30–6. 2014. doi:10.1016/j.pbb.2014.03.013. PMID 24662915.
- ↑ "Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington's disease". Hum. Mol. Genet. 22 (12): 2462–70. 2013. doi:10.1093/hmg/ddt098. PMID 23446639.
- ↑ "7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis". Neurosci. Lett. 566: 286–91. 2014. doi:10.1016/j.neulet.2014.02.058. PMID 24637017.
- ↑ "Post-injury treatment with 7,8-dihydroxyflavone, a TrkB receptor agonist, protects against experimental traumatic brain injury via PI3K/Akt signaling". PLOS ONE 9 (11): e113397. 2014. doi:10.1371/journal.pone.0113397. PMID 25415296. Bibcode: 2014PLoSO...9k3397W.
- ↑ "7,8-dihydroxyflavone, a small-molecule tropomyosin-related kinase B (TrkB) agonist, attenuates cerebral ischemia and reperfusion injury in rats". J. Mol. Histol. 45 (2): 129–40. 2014. doi:10.1007/s10735-013-9539-y. PMID 24045895.
- ↑ "TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia". CNS Neurol Disord Drug Targets 12 (3): 360–70. 2013. doi:10.2174/18715273113129990061. PMID 23469848.
- ↑ "7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome". Neuropharmacology 89: 43–53. 2015. doi:10.1016/j.neuropharm.2014.09.006. PMID 25229717.
- ↑ "7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome". J. Appl. Physiol. 112 (5): 704–10. 2012. doi:10.1152/japplphysiol.01361.2011. PMID 22194327.
- ↑ "7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats". J. Neurochem. 122 (4): 800–11. 2012. doi:10.1111/j.1471-4159.2012.07830.x. PMID 22694088.
- ↑ "7,8-Dihydroxyflavone improves memory consolidation processes in rats and mice". Behav. Brain Res. 257: 8–12. 2013. doi:10.1016/j.bbr.2013.09.029. PMID 24070857.
- ↑ "Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning". Am J Psychiatry 168 (2): 163–72. 2011. doi:10.1176/appi.ajp.2010.10030326. PMID 21123312.
- ↑ "Flavonoids, Coumarins, and Cinnamic Acids as Antioxidants in a Micellar System. Structure−Activity Relationship†". Journal of Agricultural and Food Chemistry 44 (2): 497–501. 1996. doi:10.1021/jf950378u. ISSN 0021-8561.
- ↑ "Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity". Neurosci. Lett. 499 (3): 181–5. 2011. doi:10.1016/j.neulet.2011.05.054. PMID 21651962.
- ↑ "Antioxidant action of 7,8-dihydroxyflavone protects PC12 cells against 6-hydroxydopamine-induced cytotoxicity". Neurochem. Int. 64: 18–23. 2014. doi:10.1016/j.neuint.2013.10.018. PMID 24220540.
- ↑ "Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity". Biol. Pharm. Bull. 32 (2): 166–71. 2009. doi:10.1248/bpb.32.166. PMID 19182370.
- ↑ "7,8-Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine". Psychopharmacology 231 (1): 159–66. 2014. doi:10.1007/s00213-013-3221-7. PMID 23934209.
- ↑ "Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists". Sci Signal 10 (493): eaal1670. 2017. doi:10.1126/scisignal.aal1670. PMID 28831019.
- ↑ "A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington's disease". PLOS ONE 9 (2): e87923. 2014. doi:10.1371/journal.pone.0087923. PMID 24503862. Bibcode: 2014PLoSO...987923T.
- ↑ "Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study". Environ. Health Perspect. 106 (2): 85–92. 1998. doi:10.1289/ehp.9810685. PMID 9435150.
- ↑ "Aromatase and 17beta-hydroxysteroid dehydrogenase inhibition by flavonoids". Cancer Letters 133 (1): 101–6. November 1998. doi:10.1016/S0304-3835(98)00211-0. PMID 9929167.
- ↑ "Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids". Cancer Lett. 130 (1–2): 209–16. 1998. doi:10.1016/S0304-3835(98)00141-4. PMID 9751276.
- ↑ "Flavonoids: structural requirements for antiproliferative activity on breast cancer cells". Bioorg. Med. Chem. Lett. 11 (24): 3095–7. 2001. doi:10.1016/S0960-894X(01)00617-5. PMID 11720850.
- ↑ 37.0 37.1 "A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect". Journal of Medicinal Chemistry 53 (23): 8274–86. December 2010. doi:10.1021/jm101206p. PMID 21073191.
- ↑ "7,8-Dihydroxyflavone reduces sleep during dark phase and suppresses orexin A but not orexin B in mice". Journal of Psychiatric Research 69: 110–9. October 2015. doi:10.1016/j.jpsychires.2015.08.002. PMID 26343602.
 |
