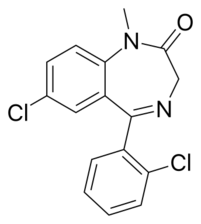
Chemistry:Diclazepam
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, sublingual |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | ~42 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C16H12Cl2N2O |
| Molar mass | 319.19 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Diclazepam (Ro5-3448), also known as chlorodiazepam and 2'-chloro-diazepam, is a benzodiazepine and functional analog of diazepam. It was first synthesized by Leo Sternbach and his team at Hoffman-La Roche in 1960.[2] It is not currently approved for use as a medication, but rather sold as an unscheduled substance.[3][4][5][6] Efficacy and safety have not been tested in humans.
In animal models, its effects are similar to diazepam, possessing long-acting anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant, and amnestic properties.[citation needed]
Metabolism
Metabolism of this compound has been assessed,[1] revealing diclazepam has an approximate elimination half-life of 42 hours and undergoes N-demethylation to delorazepam, which can be detected in urine for 6 days following administration of the parent compound.[7] Other metabolites detected were lorazepam and lormetazepam which were detectable in urine for 19 and 11 days, respectively, indicating hydroxylation by cytochrome P450 enzymes occurring concurrently with N-demethylation.
Legal status
United Kingdom
In the UK, diclazepam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other benzodiazepine drugs.[8]
United States
On December 23, 2022, the DEA announced it had begun consideration on the matter of placing Diclazepam under temporary Schedule I status.[9]
Later on July 25, 2023, the DEA published a pre-print notice that Diclazepam would become temporarily scheduled as a Schedule I controlled substance from 07/26/2023 to 07/26/2025.[10]
See also
- Diazepam
- Difludiazepam
- Delorazepam (Nordiclazepam)
- Lorazepam
- Phenazepam
- Ro09-9212
- Ro5-4864 (4'-Chlorodiazepam)
- Ro07-5220 (6'-Chlorodiclazepam)
References
- ↑ 1.0 1.1 "Characterization of the designer benzodiazepine diclazepam and preliminary data on its metabolism and pharmacokinetics". Drug Testing and Analysis 6 (7–8): 757–763. July–August 2014. doi:10.1002/dta.1628. PMID 24604775.
- ↑ "Amino substituted benzophenone oximes and derivatives thereof" US patent 3136815
- ↑ "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis 9 (4): 640–645. April 2017. doi:10.1002/dta.2003. PMID 27366870.
- ↑ "Blood concentrations of new designer benzodiazepines in forensic cases". Forensic Science International 268: 35–38. November 2016. doi:10.1016/j.forsciint.2016.09.006. PMID 27685473.
- ↑ "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis 10 (8): 1258–1269. March 2018. doi:10.1002/dta.2387. PMID 29582576. https://rke.abertay.ac.uk/en/publications/527a634d-decc-4d3a-bdca-08659bb13ed6.
- ↑ "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology 40 (2): 349–356. July 2022. doi:10.1007/s11419-022-00616-y. PMID 36454409.
- ↑ "Pharmacokinetics and bioavailability of intravenous and oral chlordesmethyldiazepam in humans". European Journal of Clinical Pharmacology 34 (1): 109–112. 1988. doi:10.1007/bf01061430. PMID 2896126.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2017". http://www.legislation.gov.uk/uksi/2017/634/contents/made.
- ↑ "(Proposed Rule) Schedules of Controlled Substances: Temporary Placement of Etizolam, Flualprazolam, Clonazolam, Flubromazolam, and Diclazepam in Schedule I". DEA. December 23, 2022. https://www.federalregister.gov/documents/2022/12/23/2022-27278/schedules-of-controlled-substances-temporary-placement-of-etizolam-flualprazolam-clonazolam.
- ↑ "Schedules of Controlled Substances: Temporary Placement of Etizolam, Flualprazolam, Clonazolam, Flubromazolam, and Diclazepam in Schedule I". DEA. July 25, 2023. https://public-inspection.federalregister.gov/2023-15748.pdf.
 |

