Chemistry:2C-T-16
| |
| Names | |
|---|---|
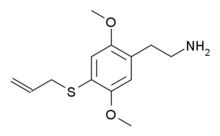
| Preferred IUPAC name
2-{2,5-Dimethoxy-4-[(prop-2-en-1-yl)sulfanyl]phenyl}ethan-1-amine | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H19NO2S | |
| Molar mass | 253.360 g/mol |
| Melting point | 193–194 °C (379–381 °F; 466–467 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2C-T-16 is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL (Phenethylamines i Have Known And Loved), however while Shulgin began synthesis of this compound he only got as far as the nitrostyrene intermediate, and did not complete the final synthetic step.[1] Synthesis of 2C-T-16 was finally achieved by Daniel Trachsel some years later,[2] and it was subsequently reported as showing similar psychedelic activity to related compounds, with a dose range of 10–25 mg and a duration of 4–6 hours,[3]:788–789 making it around the same potency as the better-known saturated analogue 2C-T-7, but with a significantly shorter duration of action. Binding studies in vitro showed 2C-T-16 to have a binding affinity of 44 nM at 5-HT2A and 15 nM at 5-HT2C.[3]:791 2C-T-16 and related derivatives are potent partial agonists of the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors and induce a head-twitch response in mice.[4]
Legality
Canada
As of October 31, 2016, 2C-T-16 is a controlled substance (Schedule III) in Canada.[5]
See also
- 2C-AL
- 2C-T-3
- 2C-T-28
- 3C-AL
- Phenethylamine
- Psychedelics, dissociatives and deliriants
References
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. http://www.erowid.org/library/books_online/pihkal/pihkal.shtml.
- ↑ Daniel Trachsel (2003). "Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines)". Helvetica Chimica Acta 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
- ↑ 3.0 3.1 Daniel Trachsel; David Lehmann; Christoph Enzensperger (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.
- ↑ Luethi D, Trachsel D, Hoener MC, Liechti ME. Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs). Neuropharmacology. 2018 May; Volume 134, Part A: 141-148.Luethi, Dino; Trachsel, Daniel; Hoener, Marius C; Liechti, Matthias E (2018). "Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs)". Neuropharmacology 134 (Pt A): 141–148. doi:10.1016/j.neuropharm.2017.07.012. PMID 28720478. https://edoc.unibas.ch/57358/1/20170920150712_59c2680084ec5.pdf.
- ↑ "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". 4 May 2016. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php.
External links
 |

