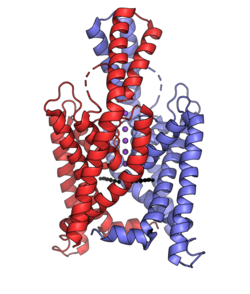
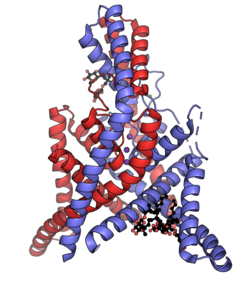
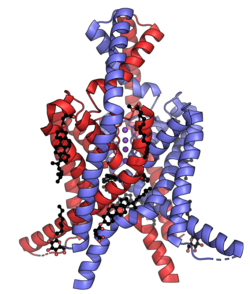
Biology:Two-pore-domain potassium channel
The two-pore-domain or tandem pore domain potassium channels are a family of 15 members that form what is known as leak channels which possess Goldman-Hodgkin-Katz (open) rectification.[1] These channels are regulated by several mechanisms including signaling lipids, oxygen tension, pH, mechanical stretch, and G-proteins.[2] Two-pore-domain potassium channels correspond structurally to a inward-rectifier potassium channel α-subunits. Each inward-rectifier potassium channel α-subunit is composed of two transmembrane α-helices, a pore helix and a potassium ion selectivity filter sequence and assembles into a tetramer forming the complete channel.[3] The two-pore domain potassium channels instead are dimers where each subunit is essentially two α-subunits joined together.[4]
Each single channel does not have two pores; the name of the channel comes from the fact that each subunit has two P (pore) domains in its primary sequence.[5] To quote Rang and Dale (2015), "The nomenclature is misleading, especially when they are incorrectly referred to as two-pore channels".[6]
Below is a list of the 15 known two-pore-domain human potassium channels:[1]
| Gene | Channel[7] | Family | Aliases |
| KCNK1 | K2p1.1 | TWIK[2][8] | TWIK-1 |
| KCNK2 | K2p2.1 | TREK[2][8] | TREK-1 |
| KCNK3 | K2p3.1 | TASK[2][8] | TASK-1 |
| KCNK4 | K2p4.1 | TREK[2][8] | TRAAK[9] |
| KCNK5 | K2p5.1 | TASK[2][8] | TASK-2[10] |
| KCNK6 | K2p6.1 | TWIK[2][8] | TWIK-2 |
| KCNK7 | K2p7.1 | TWIK[2][8] | |
| KCNK9 | K2p9.1 | TASK[2][8] | TASK-3 |
| KCNK10 | K2p10.1 | TREK[2][8] | TREK-2 |
| KCNK12 | K2p12.1 | THIK | THIK-2 |
| KCNK13 | K2p13.1 | THIK | THIK-1 |
| KCNK15 | K2p15.1 | TASK[2][8] | TASK-5 |
| KCNK16 | K2p16.1 | TALK[2][8] | TALK-1 |
| KCNK17 | K2p17.1 | TALK[2][8] | TALK-2, TASK-4 |
| KCNK18 | K2p18.1 | TRIK, TRESK[2][8][11][12] |
|
|
| ||||||||||||||||||||||||||||||||||||||||||
See also
References
- ↑ 1.0 1.1 "International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels". Pharmacological Reviews 57 (4): 527–540. December 2005. doi:10.1124/pr.57.4.12. PMID 16382106. https://escholarship.org/uc/item/3k15p5vt.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Molecular background of leak K+ currents: two-pore domain potassium channels". Physiological Reviews 90 (2): 559–605. April 2010. doi:10.1152/physrev.00029.2009. PMID 20393194. http://repo.lib.semmelweis.hu//handle/123456789/8205.
- ↑ "The structure of the potassium channel: molecular basis of K+ conduction and selectivity". Science 280 (5360): 69–77. April 1998. doi:10.1126/science.280.5360.69. PMID 9525859. Bibcode: 1998Sci...280...69D.
- ↑ "Crystal structure of the human two-pore domain potassium channel K2P1". Science 335 (6067): 432–436. January 2012. doi:10.1126/science.1213274. PMID 22282804. Bibcode: 2012Sci...335..432M.
- ↑ "Two P domain potassium channels". GtoPdb v.2023.1. IUPHAR/BPS Guide to Pharmacology. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=79.
- ↑ Pharmacology (8 ed.). Edinburgh: Churchill Livingstone. 2003. p. 59. ISBN 978-0-443-07145-4.
- ↑ "International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels". Pharmacological Reviews 55 (4): 583–586. December 2003. doi:10.1124/pr.55.4.9. PMID 14657415.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 "Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels". Cell Biochemistry and Biophysics 47 (2): 209–256. 2007. doi:10.1007/s12013-007-0007-8. PMID 17652773.
- ↑ "A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids". The EMBO Journal 17 (12): 3297–3308. June 1998. doi:10.1093/emboj/17.12.3297. PMID 9628867.
- ↑ "Potassium leak channels and the KCNK family of two-P-domain subunits". Nature Reviews. Neuroscience 2 (3): 175–184. March 2001. doi:10.1038/35058574. PMID 11256078. https://escholarship.org/uc/item/9z7112ns.
- ↑ "A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord". The Journal of Biological Chemistry 278 (30): 27406–27412. July 2003. doi:10.1074/jbc.M206810200. PMID 12754259.
- ↑ "The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin". The Journal of Biological Chemistry 279 (18): 18550–18558. April 2004. doi:10.1074/jbc.M312229200. PMID 14981085.
External links
- Tandem+Pore+Domain+Potassium+Channel at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Two-P Potassium Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=79.
 |