Chemistry:16-Ketoestradiol
From HandWiki
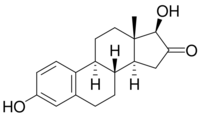
| |
| Names | |
|---|---|
| IUPAC name
3,17β-Dihydroxyestra-1,3,5(10)-trien-16-one
| |
| Systematic IUPAC name
(1R,3aS,3bR,9bS,11aS)-1,7-Dihydroxy-11a-methyl-1,3,3a,3b,4,5,9b,10,11,11a-decahydro-2H-cyclopenta[a]phenanthren-2-one | |
| Other names
17-Oxoestradiol; 17-Oxo-estradiol; 17-Keto-E2; 17-Oxo-E2; 16-Oxo-estra-1,3,5(10)-trien-3,17β-diol; NSC-51169
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H22O3 | |
| Molar mass | 286.371 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
16-Ketoestradiol (16-keto-E2, 16-oxoestradiol, or 16-oxo-E2) is an endogenous estrogen related to 16-ketoestrone.[1][2][3] 16-Ketoestrone is a very weak estrogen with only 1/1000 the estrogenic potency of estradiol in the uterus.[3] It is a so-called "short-acting" or "impeded" estrogen, similarly to estriol and dimethylstilbestrol.[4][5][6][7][8]
See also
References
- ↑ "Human Metabolome Database: Showing metabocard for 16-Ketoestradiol (HMDB0000406)". https://hmdb.ca/metabolites/HMDB0000406.
- ↑ Breuer, Heinz (1962). The Metabolism of the Natural Estrogens. Vitamins & Hormones. 20. pp. 285–335. doi:10.1016/S0083-6729(08)60720-7. ISBN 9780127098203.
- ↑ 3.0 3.1 "The depression of estrone-induced uterine growth by phenolic estrogens with oxygenated functions at positions 6 or 16: the impeded estrogens". J. Exp. Med. 102 (3): 335–46. September 1955. doi:10.1084/jem.102.3.335. PMID 13252187.
- ↑ "Nuclear binding and retention of the receptor estrogen complex: relation to the agonistic and antagonistic properties of estriol". Endocrinology 100 (1): 91–6. January 1977. doi:10.1210/endo-100-1-91. PMID 830547.
- ↑ "Mechanism of action of estrogen agonists and antagonists". J. Anim. Sci. 49 (Suppl 2): 46–65. 1979. doi:10.1093/ansci/49.supplement_ii.46. PMID 400777.
- ↑ "Antioestrogens. A review". Clin. Endocrinol. (Oxf) 4 (5): 551–72. September 1975. doi:10.1111/j.1365-2265.1975.tb01568.x. PMID 170029.
- ↑ "The agonistic and antagonistic effects of short acting estrogens: a review". Pharmacol. Ther. 21 (3): 429–53. 1983. doi:10.1016/0163-7258(83)90063-3. PMID 6356176.
- ↑ "The agonistic and antagonistic actions of estriol". J. Steroid Biochem. 20 (4B): 1005–13. April 1984. doi:10.1016/0022-4731(84)90011-6. PMID 6202959.
 |

