Chemistry:Esmethadone
From HandWiki
Short description: (S)-enantiomer of methadone
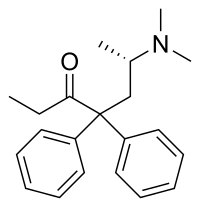 | |
| Clinical data | |
|---|---|
| Other names | Dextromethadone; d-Methadone; 6S-Methadone; (+)-Methadone |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C21H27NO |
| Molar mass | 309.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Esmethadone (INN; developmental code name REL-1017), also known as dextromethadone, is the (S)-enantiomer of methadone. It acts as an N-methyl-D-aspartate receptor (NMDAR) antagonist, among other actions.[1] Unlike levomethadone, it has low affinity for opioid receptors and lacks significant respiratory depressant action and abuse liability.[2][3] Esmethadone is under development for the treatment of major depressive disorder.[4] As of August 2022, it is in phase 3 clinical trials for this indication.[4]
There is an asymmetric synthesis available to prepare both esmethadone (S-(+)-methadone) and levomethadone (R-(−)-methadone).[5][6]
References
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9058409 - ↑ "METHADONE". Drug Enforcement Agency. https://www.deadiversion.usdoj.gov/drug_chem_info/methadone/methadone.pdf#search=methadone.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid7562497 - ↑ 4.0 4.1 "Dextromethadone - Cornell University/Relmada Therapeutics - AdisInsight". https://adisinsight.springer.com/drugs/800038927.
- ↑ "Synthesis of optically active methadones, LAAM and bufuralol by lipase-catalysed acylations". Tetrahedron: Asymmetry 14 (5): 567–576. March 2003. doi:10.1016/S0957-4166(03)00019-3.
- ↑ US patent 6143933
 | 0.00      (0 votes) (0 votes) |
