Chemistry:NPDPA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
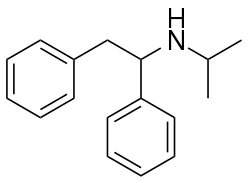
| Formula | C17H21N |
| Molar mass | 239.362 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
NPDPA (also known as isopropylphenidine or isophenidine) is a dissociative anesthetic that has been sold online as a designer drug.[1][2][3][4] It was first identified in Germany in 2008, and while it has never been as widely sold as related compounds such as diphenidine and ephenidine, it has continued to show up in seized drug samples occasionally,[5] and was banned in Sweden in 2015.
Metabolism
Isopropylphenidine's metabolic pathway consists of N-oxidation, N-dealkylation, mono- and bis-hydroxylation of the benzene ring, and hydroxylation of the phenyl ring only after N-dealkylation. The dihydroxy metabolites were conjugated by methylation of one hydroxy group, and hydroxy metabolites by glucuronidation or sulfation.[3][6]
Legality
Sweden's public health agency suggested that NPDPA be classified as a hazardous substance on 1 June 2015. Due to that suggestion it became a scheduled substance in Sweden, as of 18 August 2015.[7] It has also been proposed for control in Germany under analogue provisions, though these have not yet come into force as of 2016.
See also
References
- ↑ "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis 6 (7–8): 614–32. July–August 2014. doi:10.1002/dta.1620. PMID 24678061.
- ↑ "Michaelis-Menten kinetic analysis of drugs of abuse to estimate their affinity to human P-glycoprotein". Toxicology Letters 217 (2): 137–42. February 2013. doi:10.1016/j.toxlet.2012.12.012. PMID 23273999.
- ↑ 3.0 3.1 "Lefetamine-derived designer drugs N-ethyl-1,2-diphenylethylamine (NEDPA) and N-iso-propyl-1,2-diphenylethylamine (NPDPA): metabolism and detectability in rat urine using GC-MS, LC-MSn and LC-HR-MS/MS". Drug Testing and Analysis 6 (10): 1038–48. October 2014. doi:10.1002/dta.1621. PMID 24591097.
- ↑ "Toxicokinetics of lefetamine and derived diphenylethylamine designer drugs-Contribution of human cytochrome P450 isozymes to their main phase I metabolic steps". Toxicology Letters 238 (3): 39–44. November 2015. doi:10.1016/j.toxlet.2015.08.012. PMID 26276083.
- ↑ "An overview of emerging and new psychoactive substances in the United Kingdom". Forensic Science International 267: 25–34. October 2016. doi:10.1016/j.forsciint.2016.08.013. PMID 27552699. https://discovery.ucl.ac.uk/id/eprint/1508803/7/Gibbons_FSI-D-16-00226R1_extracted.pdf.
- ↑ "Lefetamine, a controlled drug and pharmaceutical lead of new designer drugs: synthesis, metabolism, and detectability in urine and human liver preparations using GC-MS, LC-MS(n), and LC-high resolution-MS/MS". Analytical and Bioanalytical Chemistry 407 (6): 1545–57. February 2015. doi:10.1007/s00216-014-8414-3. PMID 25577353.
- ↑ "23 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in sv). Folkhälsomyndigheten. 1 June 2015. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2015/juni/23-nya-amnen-kan-klassas-som-narkotika-eller-halsofarlig-vara.
 |
