Chemistry:Tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men.[13] It is also being studied for other types of cancer.[13] It has been used for Albright syndrome.[14] Tamoxifen is typically taken daily by mouth for five years for breast cancer.[14]
Serious side effects include a small increased risk of uterine cancer, stroke, vision problems, and pulmonary embolism.[14] Common side effects include irregular periods, weight loss, and hot flashes.[14] It may cause harm to the baby if taken during pregnancy or breastfeeding.[14] It is a selective estrogen-receptor modulator (SERM) and works by decreasing the growth of breast cancer cells.[14][15] It is a member of the triphenylethylene group of compounds.[16]
Tamoxifen was initially made in 1962, by chemist Dora Richardson.[17][18] It is on the World Health Organization's List of Essential Medicines.[19] Tamoxifen is available as a generic medication.[14] In 2020, it was the 317th most commonly prescribed medication in the United States, with more than 900 thousand prescriptions.[20][21]
Medical uses
Dysmenorrhea
Tamoxifen has been used effectively to improve blood flow, reduce uterine contractility and pain in dysmenorrhea patients.[22]
Breast cancer
Tamoxifen is used for the treatment of both early and advanced estrogen receptor-positive (ER-positive or ER+) breast cancer in pre- and postmenopausal women.[23] Tamoxifen increases the risk of postmenopausal bleeding, endometrial polyps, hyperplasia, and endometrial cancer; using tamoxifen with an intrauterine system releasing levonorgestrel might increase vaginal bleeding after 1 to 2 years, but reduces somewhat endometrial polyps and hyperplasia, but not necessarily endometrial cancer.[24] Additionally, it is the most common hormone treatment for male breast cancer.[25] It is also approved by the FDA for the prevention of breast cancer in women at high risk of developing the disease.[26] It has been further approved for the reduction of contralateral (in the opposite breast) cancer. The use of tamoxifen is recommended for 10 years.[27]
In 2006, the large STAR clinical study concluded that raloxifene is also effective in reducing the incidence of breast cancer. Updated results after an average of 6.75 years of follow up found that raloxifene retains 76% of tamoxifen's effectiveness in preventing invasive breast cancer, with 45% fewer uterine cancers and 25% fewer blood clots in women taking raloxifene than in women taking tamoxifen.[28][29][30]
Infertility
Tamoxifen is used for ovulation induction to treat infertility in women with anovulatory disorders. It is given at days three to seven of a woman's cycle.[31]
Tamoxifen improves fertility in males with infertility by disinhibiting the hypothalamic–pituitary–gonadal axis (HPG axis) via ER antagonism and thereby increasing the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and increasing testicular testosterone production.[32]
Gynecomastia
Tamoxifen is used to prevent and treat gynecomastia.[33][34] It is taken as a preventative measure in small doses, or used at the onset of any symptoms such as nipple soreness or sensitivity. Other medications are taken for similar purposes such as clomifene and the anti-aromatase drugs which are used in order to try to avoid the hormone-related adverse effects.
Early puberty
Tamoxifen is useful in the treatment of peripheral precocious puberty, for instance due to McCune–Albright syndrome, in both girls and boys.[35][36][37] It has been found to decrease growth velocity and the rate of bone maturation in girls with precocious puberty, and hence to improve final height in these individuals.[35][36]
Available forms
Tamoxifen is available as a tablet or oral solution.[38][39]
Contraindications
Tamoxifen has a number of contraindications, including known hypersensitivity to tamoxifen or other ingredients, individuals taking concomitant coumarin-type anticoagulant therapy, and women with a history of venous thromboembolism (deep vein thrombosis or pulmonary embolism).[12]
Side effects
A report in September 2009 from Health and Human Services' Agency for Healthcare Research and Quality suggests that tamoxifen, raloxifene, and tibolone used to treat breast cancer significantly reduce invasive breast cancer in midlife and older women, but also increase the risk of adverse side effects.[40]
Endometrial cancer
Tamoxifen is a selective estrogen receptor modulator (SERM).[41] Even though it is an antagonist in breast tissue it acts as partial agonist on the endometrium and has been linked to endometrial cancer in some women. Therefore, endometrial changes, including cancer, are among tamoxifen's side effects.[42] With time, risk of endometrial cancer may be doubled to quadrupled, which is a reason tamoxifen is typically only used for five years.[43]
The American Cancer Society lists tamoxifen as a known carcinogen, stating that it increases the risk of some types of uterine cancer while lowering the risk of breast cancer recurrence.[44]
Cardiovascular and metabolic
Tamoxifen treatment of postmenopausal women is associated with beneficial effects on serum lipid profiles. However, long-term data from clinical trials have failed to demonstrate a cardioprotective effect.[45] For some women, tamoxifen can cause a rapid increase in triglyceride concentration in the blood. In addition, there is an increased risk of thromboembolism especially during and immediately after major surgery or periods of immobility.[46] Use of tamoxifen has been shown to slightly increase risk of deep vein thrombosis, pulmonary embolism, and stroke.[47]
Liver toxicity
Tamoxifen has been associated with a number of cases of hepatotoxicity.[48] Several different varieties of hepatotoxicity have been reported.[48] Tamoxifen can also precipitate non-alcoholic fatty liver disease in obese and overweight women (not in normal weight women) at an average rate of 40% after a year use with 20 mg/day.[49]
Overdose
Acute overdose of tamoxifen has not been reported in humans.[12] In dose-ranging studies, tamoxifen was administered at very high doses in women (e.g., 300 mg/m2) and was found to produce acute neurotoxicity including tremor, hyperreflexia, unsteady gait, and dizziness.[12] These symptoms occurred within three to five days of therapy and disappeared within two to five days of discontinuation of therapy.[12] No indications of permanent neurotoxicity were observed.[12] QT prolongation was also observed with very high doses of tamoxifen.[12] There is no specific antidote for overdose of tamoxifen.[12] Instead, treatment should be based on symptoms.[12]
Interactions
Patients with variant forms of the gene CYP2D6 may not receive full benefit from tamoxifen because of too slow metabolism of the tamoxifen prodrug into its active metabolites.[50][51] On 18 October 2006, the Subcommittee for Clinical Pharmacology recommended relabeling tamoxifen to include information about this gene in the package insert.[52] Certain CYP2D6 variations in breast cancer patients lead to a worse clinical outcome for tamoxifen treatment.[53] Genotyping therefore has the potential for identification of women who have these CYP2D6 phenotypes and for whom the use of tamoxifen is associated with poor outcomes. Recent research has shown that 7–10% of women with breast cancer may not receive the full medical benefit from taking tamoxifen due to their genetic make-up. DNA Drug Safety Testing can examine DNA variations in the CYP2D6 and other important drug processing pathways. More than 20% of all clinically used medications are metabolized by CYP2D6 and knowing the CYP2D6 status of a person can help the doctor with the future selection of medications.[54] Other molecular biomarkers may also be used to select appropriate patients likely to benefit from tamoxifen.[55]
Recent studies suggest that taking the selective serotonin reuptake inhibitors (SSRIs) antidepressants paroxetine (Paxil), fluoxetine (Prozac), and sertraline (Zoloft) can decrease the effectiveness of tamoxifen, as these drugs compete for the CYP2D6 enzyme which is needed to metabolize tamoxifen into its active forms.[56] A U.S. study presented at the American Society of Clinical Oncology's annual meeting in 2009 found that after two years, 7.5% of women who took only tamoxifen had a recurrence, compared with 16% who took either paroxetine, fluoxetine or sertraline, drugs considered to be the most potent CYP2D6 inhibitors. That difference translates to a 120% increase in the risk of breast cancer recurrence. Patients taking the SSRIs Celexa (citalopram), Lexapro (escitalopram), and Luvox (fluvoxamine) did not have an increased risk of recurrence, due to their lack of competitive metabolism for the CYP2D6 enzyme.[57] A newer study demonstrated a clearer and stronger effect from paroxetine in causing the worst outcomes. Patients treated with both paroxetine and tamoxifen have a 67% increased risk of death from breast cancer, from 24% to 91%, depending on the duration of coadministration.[58]
Tamoxifen interacts with certain other antiestrogens.[5] The aromatase inhibitor aminoglutethimide induces the metabolism of tamoxifen.[5] Conversely, the aromatase inhibitor letrozole does not affect the metabolism of tamoxifen.[5] However, tamoxifen induces the metabolism of letrozole and significantly reduces its concentrations.[5]
Pharmacology
Pharmacodynamics
Selective estrogen receptor modulator activity
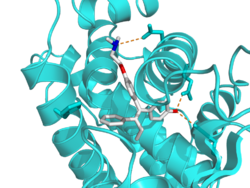
Tamoxifen acts as a selective estrogen receptor modulator (SERM), or as a partial agonist of the estrogen receptors (ERs). It has mixed estrogenic and antiestrogenic activity, with its profile of effects differing by tissue. For instance, tamoxifen has predominantly antiestrogenic effects in the breasts but predominantly estrogenic effects in the uterus and liver. In breast tissue, tamoxifen acts as an ER antagonist so that transcription of estrogen-responsive genes is inhibited.[60] A beneficial side effect of tamoxifen is that it prevents bone loss by acting as an ER agonist (i.e., mimicking the effects of estrogen) in this cell type. Therefore, by inhibiting osteoclasts, it prevents osteoporosis.[61][62] When tamoxifen was launched as a drug, it was thought that tamoxifen would act as an ER antagonist in all tissues, including bone, and therefore it was feared that it would contribute to osteoporosis. It was therefore very surprising that the opposite effect was observed clinically. Hence tamoxifen's tissue selective action directly led to the formulation of the concept of SERMs.[63]
| Medication | Breast | Bone | Liver | Uterus | Vagina | Brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipids | Coagulation | SHBG | IGF-1 | Hot flashes | Gonadotropins | |||||||||
| Estradiol | + | + | + | + | + | + | + | + | + | + | ||||
| "Ideal SERM" | – | + | + | ± | ± | ± | – | + | + | ± | ||||
| Bazedoxifene | – | + | + | + | + | ? | – | ± | – | ? | ||||
| Clomifene | – | + | + | ? | + | + | – | ? | – | ± | ||||
| Lasofoxifene | – | + | + | + | ? | ? | ± | ± | – | ? | ||||
| Ospemifene | – | + | + | + | + | + | ± | ± | – | ± | ||||
| Raloxifene | – | + | + | + | + | + | ± | – | – | ± | ||||
| Tamoxifen | – | + | + | + | + | + | + | – | – | ± | ||||
| Toremifene | – | + | + | + | + | + | + | – | – | ± | ||||
| Effect: + = Estrogenic / agonistic. ± = Mixed or neutral. – = Antiestrogenic / antagonistic]]. Sources: See template. | ||||||||||||||
Tamoxifen is a long-acting SERM, with a nuclear retention of the ER–tamoxifen (or metabolite) complex of greater than 48 hours.[64][65] It has relatively little affinity for the ERs itself and instead acts as a prodrug of active metabolites such as endoxifen (4-hydroxy-N-desmethyltamoxifen) and afimoxifene (4-hydroxytamoxifen; 4-OHT).[9] These metabolites have approximately 30 to 100 times greater affinity for the ERs than tamoxifen itself.[8][66] Per one study, tamoxifen had 7% and 6% of the affinity of estradiol for the ERα and ERβ, respectively, whereas afimoxifene had 178% and 338% of the affinity of estradiol for the ERα and ERβ, respectively.[67] Hence, afimoxifene showed 25-fold higher affinity for the ERα and 56-fold higher affinity for the ERβ than tamoxifen.[68] The antiestrogenic potencies of endoxifen and afimoxifene are very similar.[9] However, endoxifen occurs in much higher concentrations than afimoxifene and is now thought to be the major active form of tamoxifen in the body.[8][9][69]
Tamoxifen binds to ER competitively (with respect to the endogenous agonist estrogen) in tumor cells and other tissue targets, producing a nuclear complex that decreases DNA synthesis and inhibits estrogen effects. It is a nonsteroidal agent with potent antiestrogenic properties which compete with estrogen for binding sites in breast and other tissues. Tamoxifen causes cells to remain in the G0 and G1 phases of the cell cycle. Because it prevents (pre)cancerous cells from dividing but does not cause cell death, tamoxifen is cytostatic rather than cytocidal. Tamoxifen binds to ER, the ER/tamoxifen complex recruits other proteins known as co-repressors, and the complex then binds to DNA to modulate gene expression. Some of these proteins include NCoR and SMRT.[70] Tamoxifen function can be regulated by a number of different variables including growth factors.[71] Tamoxifen needs to block growth factor proteins such as ErbB2/HER2[72] because high levels of ErbB2 have been shown to occur in tamoxifen resistant cancers.[73] Tamoxifen seems to require a protein PAX2 for its full anticancer effect.[72][74] In the presence of high PAX2 expression, the tamoxifen/ER complex is able to suppress the expression of the pro-proliferative ERBB2 protein. In contrast, when AIB-1 expression is higher than PAX2, tamoxifen/ER complex upregulates the expression of ERBB2 resulting in stimulation of breast cancer growth.[72][75]
Tamoxifen is antigonadotropic in postmenopausal women and partially suppresses levels of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in such women.[76] However, it has progonadotropic effects in premenopausal women and increases estrogen levels by 6-fold in them.[76] Due to the nature of tamoxifen as a competitive ER ligand, this increase in estrogen levels is liable to interfere with the antiestrogenic efficacy of tamoxifen.[76] The effects of tamoxifen on breast cancer Ki-67 expression, sex hormone-binding globulin (SHBG) levels, and IGF-1 levels are dose-dependent across a dosage range of 1 to 20 mg/day in women with breast cancer.[77] Tamoxifen has been found to decrease insulin-like growth factor 1 (IGF-1) levels by 17 to 38% in women and men.[78] Suppression of IGF-1 production in the liver is a well-known action of estrogens and SERMs.[78] A 10 mg/day dosage of tamoxifen is nearly as effective as a 20 mg/day dosage in suppressing IGF-1 levels.[5]
Other activities
Afimoxifene is an agonist of the G protein-coupled estrogen receptor (GPER) with relatively low affinity.[79] Its affinity for the receptor is in the range of 100 to 1,000 nM, relative to 3 to 6 nM for estradiol.[79]
In addition to its activity as a SERM, afimoxifene binds to both the estrogen-related receptor β and estrogen-related receptor γ and is an antagonist of the estrogen-related receptor γ (ERRγ).[80]
Norendoxifen (4-hydroxy-N,N-didesmethyltamoxifen), another active metabolite of tamoxifen, has been found to act as a potent competitive aromatase inhibitor (IC50 = 90 nM), and may also be involved in the antiestrogenic activity of tamoxifen.[81]
In addition to its activity as a SERM, tamoxifen is a potent and selective protein kinase C inhibitor, and is active in this regard at therapeutic concentrations.[82] This action is thought to underlie the efficacy of tamoxifen in the treatment of bipolar disorder.[82]
Tamoxifen is an inhibitor of P-glycoprotein.[12]
Pharmacokinetics
Absorption
Tamoxifen is rapidly and extensively absorbed from the intestines with oral administration.[5][6] The oral bioavailability of tamoxifen is approximately 100%, which is suggestive of minimal first-pass metabolism in the intestines and liver.[5] Following intake, peak levels of tamoxifen occur after three to seven hours.[83][5] Steady state levels of tamoxifen are reached typically after 3 to 4 weeks but possibly up to 16 weeks of daily administration.[5][11] Steady state levels of afimoxifene are achieved after 8 weeks of daily tamoxifen administration.[11][7] Peak levels of tamoxifen after a single 40 mg oral dose were 65 ng/mL and steady state levels at 20 mg/day were 310 ng/mL.[5] Levels of tamoxifen show clear dose dependency across a dosage range of 1 to 20 mg/day.[5][84] Endoxifen levels are approximately 5 to 10 times higher than afimoxifene levels, with large interindividual variability.[8][9] Endoxifen levels have been reported as 10.8 to 15.9 ng/mL at steady state in CYP2D6 normal metabolizers during therapy with 20 mg/day tamoxifen.[8] The most abundant metabolites of tamoxifen in terms of circulating concentrations are N-desmethyltamoxifen, N,N-didesmethyltamoxifen, (Z)-endoxifen, and tamoxifen N-oxide.[10][85]
Distribution
The volume of distribution of tamoxifen is 50 to 60 L/kg and its clearance has been estimated as 1.2 to 5.1 L/hour.[5][83] High concentrations of tamoxifen have been found in breast, uterus, liver, kidney, lung, pancreas, and ovary tissue in animals and humans.[5] Levels of tamoxifen in the uterus have been found to be 2- to 3-fold higher than in the circulation[5] and in the breasts 10-fold higher than in the circulation.[84] The plasma protein binding of tamoxifen and afimoxifene is greater than 99%.[7] A majority of tamoxifen is bound to albumin.[5] Albumin alone binds 98.8% of tamoxifen while other plasma proteins are not greatly involved.[86]
Metabolism
| Compound | Mean plasma concentrations |
Effect on ER / affinity for ERa |
|---|---|---|
| Tamoxifen | 190–420 nmol/L | Weak antagonist / 2% |
| N-Desmethyltamoxifen | 280–800 nmol/L | Weak antagonist / 1% |
| N,N-Desmethyltamoxifen | 90–120 nmol/L | Weak antagonist |
| Endoxifen | 14–130 nmol/L | Strong antagonist / equal to afimoxifene |
| Afimoxifene | 3–17 nmol/Lb | Strong antagonist / 188% |
| α-Hydroxytamoxifen | 1 nmol/L | None |
| 3,4-Dihydroxytamoxifen | ? | Weak antagonist / high affinity |
| Tamoxifen N-oxide | 15–24 nmol/L | Weak antagonistc |
| Footnotes: a = Estradiol is 100%. b = One study reported a much higher concentration (67 nmol/L). c = Might be due to reduction to tamoxifen. | ||
Tamoxifen is a prodrug and is metabolized in the liver by the cytochrome P450 isoforms CYP3A4, CYP2C9, and CYP2D6 into active metabolites such as endoxifen (4-hydroxy-N-desmethyltamoxifen) and afimoxifene (4-hydroxytamoxifen).[5][12][8] Conversion of tamoxifen by N-demethylation into N-desmethyltamoxifen, which is catalyzed primarily by CYP3A4 and CYP3A5, is responsible for approximately 92% of tamoxifen metabolism.[9] Conversely, 4-hydroxylation of tamoxifen into afimoxifene is responsible for only about 7% of tamoxifen metabolism.[9] Following its formation, N-desmethyltamoxifen is oxidized into several other metabolites, the most notable of which is endoxifen.[9] Another active metabolite, norendoxifen (4-hydroxy-N,N-didesmethyltamoxifen), is formed via N-demethylation of endoxifen or 4-hydroxylation of N,N-didesmethyltamoxifen.[8] Tamoxifen and its metabolites undergo conjugation, including glucuronidation and sulfation.[11] Tamoxifen may inhibit its own metabolism.[5]
Elimination
Tamoxifen has a long elimination half-life of typically 5 to 7 days, with a range of 4 to 11 days.[5][8][83] Similarly, the half-life of afimoxifene is 14 days.[7] Conversely, the half-life of endoxifen is 50 to 70 hours (2–3 days).[8] The long half-lives of tamoxifen and afimoxifene are attributed to their high plasma protein binding as well as to enterohepatic recirculation.[7] Upon discontinuation of treatment, levels of tamoxifen and its metabolites persist in the circulation for at least 6 weeks.[7] Tamoxifen is excreted in bile and is eliminated in feces, while small amounts are eliminated in urine.[5]
Chemistry
Tamoxifen is a nonsteroidal SERM of the triphenylethylene family and was structurally derived from diethylstilbestrol-like estrogens and antiestrogens such as chlorotrianisene and ethamoxytriphetol.[88][89][90][91] Initially, clomifene was synthesized, and tamoxifen was developed subsequently.[88][90][91] Tamoxifen is closely related structurally to other triphenylethylenes, such as clomifene, nafoxidine, ospemifene, toremifene, and numerous others.[92][93] Other SERMs, like raloxifene, are structurally distinct from tamoxifen and other triphenylethylenes.[93]
History
In the late 1950s, pharmaceutical companies were actively researching a newly discovered class of anti-estrogen compounds in the hope of developing a morning-after contraceptive pill. Arthur L Walpole was a reproductive endocrinologist who led such a team at the Alderley Park research laboratories of ICI Pharmaceuticals.[18] It was there in 1962 that chemist Dora Richardson first synthesized tamoxifen, back then known as ICI-46,474, when she was looking to create triphenylethylene derivatives for the contraceptive pill project that her team was researching.[94]
This compound was originally created to work as an estrogen inhibitor, but instead was found to stimulate ovulation in participants of the drug testing trial.[17] Walpole and his colleagues filed a UK patent covering this compound in 1962, but patent protection on this compound was repeatedly denied in the US until the 1980s.[95] Tamoxifen did eventually receive marketing approval as a fertility treatment, but the class of compounds never proved useful in human contraception. A link between estrogen and breast cancer had been known for many years, but cancer treatments were not a corporate priority at the time, and Walpole's personal interests were important in keeping support for the compound alive in the face of this and the lack of patent protection.[18] It was only when Walpole threatened to leave his position that corporate decided to allow trials and testing for tamoxifen as a drug that could be used to treat breast cancer. Without Walpole's effort towards defending the work that his team had done in discovering a possibly revolutionary source for breast cancer treatment, tamoxifen could have become a discarded or under-researched idea. Walpole's team consisted of Dora Richardson and G. A. Snow, who worked on the chemistry portion of the project, along with G. E. Paget and J. K. Walley, who focused primarily on the biological side.[17]
Tamoxifen is one of three drugs in an anti-angiogenetic protocol developed by Dr. Judah Folkman, a researcher at Children's Hospital at Harvard Medical School in Boston. Folkman discovered in the 1970s that angiogenesis – the growth of new blood vessels – plays a significant role in the development of cancer. Since his discovery, an entirely new field of cancer research has developed. Clinical trials on angiogenesis inhibitors have been underway since 1992 using many different drugs. The Harvard researchers developed a specific protocol for a golden retriever named Navy who was cancer-free after receiving the prescribed cocktail of celecoxib, doxycycline, and tamoxifen – the treatment subsequently became known as the Navy Protocol.[96] Furthermore, tamoxifen treatment alone has been shown to have anti-angiogenetic effects in animal models of cancer which appear to be, at least in part, independent of tamoxifen's ER antagonist properties.[97]
Other antiestrogens, such as ethamoxytriphetol (MER-25) and clomifene (MRL-41), were assessed for treatment of breast cancer and found to be effective before tamoxifen, but were plagued with toxicity issues.[98][99] The first clinical study of tamoxifen took place at the Christie Hospital in 1971, and showed a convincing effect in advanced breast cancer, but nevertheless ICI's development programme came close to termination when it was reviewed in 1972.[100] In an unpublished article from the early days of the trial, Dora Richardson documented her team's excitement about tamoxifen's effects in counteracting infertility problems and the early positive effects found in breast cancer patients. Unfortunately, this work was not well received by everyone, as the team was supposed to be looking for a contraceptive pill.[17] Tamoxifen's further development may have been bolstered by a second clinical study by Harold W.C. Ward [101] at the Queen Elizabeth Hospital, Birmingham. Ward's study showed a more definitive response to the drug at a higher dosage. Walpole also may have helped to convince the company to market tamoxifen for late stage breast cancer in 1973.[95] He was also instrumental in funding V. Craig Jordan to work on tamoxifen. In 1972, ICI Pharmaceuticals Division abandoned development of tamoxifen for financial reasons. The drug was subsequently reinvented from a failed contraceptive, to become tamoxifen, the gold standard for the adjuvant treatment of breast cancer and the pioneering medicine for chemprevention for high-risk women.[102][103] Two books, Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health (Imperial College Press 2013) and Tamoxifen: Pioneering Medicine in Breast Cancer (Springer 2013) tell this story.
1980 saw the publication of the first trial to show that tamoxifen given in addition to chemotherapy improved survival for patients with early breast cancer.[104] In advanced disease, tamoxifen is now only recognized as effective in ER+ patients, but the early trials did not select ER+ patients, and by the mid-1980s the clinical trial picture was not showing a major advantage for tamoxifen.[105] Nevertheless, tamoxifen had a relatively mild side-effect profile, and a number of large trials continued.
The pharmacology of SERMs was discovered, defined, and deciphered during the 1980s.[106] A clinical strategy was described[107] that led to the creation of SERMs as a group of multifunctional medicines aimed at the treatment or prevention of many conditions in postmenopausal women, e.g. osteoporosis and breast cancer. This story is told in: V. Craig Jordan, ed. 2013. "Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health" Imperial College Press, Singapore.
The early sales of tamoxifen in both the UK and in the U.S. far exceeded ICI's original estimate, but despite this, at the annual portfolio review ICI's board members still asserted that "there was no market for cancer", leaving the drug's marketing success to rely on its clinical results and clinicians' and scientists' interests in it. Shortly after, Dora Richardson published a history of tamoxifen that, unusually for that type of paper, included personal accounts and letters from patients who attributed their healing to the drug. It is by giving voice to cancer patients using tamoxifen, and so helping to push it forward, by justifying it both morally and scientifically to corporations.[17]
It was not until 1998 that the meta-analysis of the Oxford-based Early Breast Cancer Trialists' Collaborative Group showed definitively that tamoxifen was effective for early breast cancer.[108]
Society and culture
Brand names
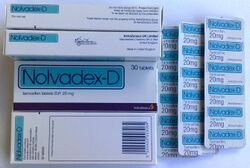
Tamoxifen is marketed under the brand names Nolvadex and Soltamox, and a variety of other brand names throughout the world.[1][109]
Economics
Global sales of tamoxifen in 2001 were approximately $1.02 billion.[110] Since the expiration of the patent in 2002, it is widely available as a generic drug around the world. (As of 2004), tamoxifen was the world's largest selling hormonal drug for the treatment of breast cancer.[111]
Research
In McCune-Albright syndrome (MAS) tamoxifen has been used to treat premature puberty and the consequences of premature puberty. Tamoxifen has been seen to decrease rapid bone maturation which is the result of excessive estrogen and alter predicted adult height (PAH).[112][113] The same effects have also been seen in short pubertal boys.[114] However, one in vitro study in 2007 and later an in vivo study in 2008 have shown that tamoxifen induces apoptosis in growth plate chondrocytes, reduces serum insulin-like growth factor 1 (IGF-1) levels and causes persistent retardation of longitudinal and cortical radial bone growth in young male rats, leading the researchers to express concern giving tamoxifen to growing individuals.[115][116]
Tamoxifen has been studied in the treatment of the rare conditions of retroperitoneal fibrosis[117] and idiopathic sclerosing mesenteritis.[118] It has also been proposed as part of a treatment plan for Riedel's thyroiditis.[119]
Tamoxifen is used as a research tool to trigger tissue-specific gene expression in many conditional expression constructs in genetically modified animals including a version of the Cre-Lox recombination technique.[120] While widely used in transgenic research, the strong anabolic effect of tamoxifen on bone might confound this approach, especially as it relates to bone-targeted constructs.
Tamoxifen may be effective in the treatment of mania in people with bipolar disorder.[121] This is thought to be due to blockade of protein kinase C (PKC), an enzyme that regulates neuron activity in the brain.[121][122] Researchers believe PKC is overactive during the mania in bipolar patients.[121][122] (As of September 2019), endoxifen, a major active metabolite of tamoxifen with a 4-fold more potent PKC inhibition, was in phase III clinical trials for bipolar disorder.[123][124]
References
- ↑ 1.0 1.1 "NCI Drug Dictionary". 2 February 2011. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/tamoxifen-citrate.
- ↑ "Tamoxifen Use During Pregnancy". 25 July 2019. https://www.drugs.com/pregnancy/tamoxifen.html.
- ↑ "Tamoxifen citrate tablet, film coated". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c82205a3-ae0c-4ce5-b04b-9581cd8c70d4.
- ↑ "Soltamox- tamoxifen citrate liquid". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6ff055-590c-41e6-9530-1fdf04cdbd02.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 "Pharmacokinetics of selective estrogen receptor modulators". Clinical Pharmacokinetics 42 (4): 361–372. 2003. doi:10.2165/00003088-200342040-00004. PMID 12648026.
- ↑ 6.0 6.1 Brenner and Stevens' Pharmacology E-Book. Elsevier Health Sciences. 28 September 2017. pp. 394–. ISBN 978-0-323-39172-6. https://books.google.com/books?id=v3g4DwAAQBAJ&pg=PA394.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. 7 December 2011. pp. 655–. ISBN 978-1-4511-4820-6. https://books.google.com/books?id=0U4aj4GZWCIC&pg=PA655.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 8.15 "Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen". Expert Review of Clinical Pharmacology 12 (6): 523–536. June 2019. doi:10.1080/17512433.2019.1610390. PMID 31008668.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 "PharmGKB summary: tamoxifen pathway, pharmacokinetics". Pharmacogenetics and Genomics 23 (11): 643–647. November 2013. doi:10.1097/FPC.0b013e3283656bc1. PMID 23962908.
- ↑ 10.0 10.1 "Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma". Clinical Pharmacology and Therapeutics 89 (5): 708–717. May 2011. doi:10.1038/clpt.2011.27. PMID 21451508.
- ↑ 11.0 11.1 11.2 11.3 "Pharmacokinetics of Anti-Cancer Drugs Used in Breast Cancer Chemotherapy". Chemo Fog. Advances in Experimental Medicine and Biology. 678. 2010. pp. 124–132. doi:10.1007/978-1-4419-6306-2_16. ISBN 978-1-4419-6305-5.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 "Nolvadex (Tamoxifen Citrate) tablets". 3 November 2016. https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=234988.
- ↑ 13.0 13.1 "Tamoxifen Citrate". 26 August 2015. http://www.cancer.gov/about-cancer/treatment/drugs/tamoxifencitrate.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 "Tamoxifen Citrate". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/tamoxifen-citrate.html.
- ↑ "Selective estrogen receptor modulators". https://www.drugs.com/drug-class/selective-estrogen-receptor-modulators.html.
- ↑ Selective Estrogen Receptor Modulators a New Brand of Multitarget Drugs. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg. 2006. p. 52. ISBN 9783540347422. https://books.google.com/books?id=heJDAAAAQBAJ&pg=PA52.
- ↑ 17.0 17.1 17.2 17.3 17.4 "Tamoxifen from Failed Contraceptive Pill to Best-Selling Breast Cancer Medicine: A Case-Study in Pharmaceutical Innovation". Frontiers in Pharmacology 8: 620. 12 September 2017. doi:10.3389/fphar.2017.00620. PMID 28955226.
- ↑ 18.0 18.1 18.2 "Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer". British Journal of Pharmacology 147 (Suppl 1): S269–S276. January 2006. doi:10.1038/sj.bjp.0706399. PMID 16402113.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Tamoxifen Citrate - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/TamoxifenCitrate.
- ↑ "Modern management of dysmenorrhoea". Trends in Urology, Gynaecology & Sexual Health 14 (5): 25–29. 2009. doi:10.1002/tre.120.
- ↑ "Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer". British Journal of Pharmacology 110 (2): 507–517. October 1993. doi:10.1111/j.1476-5381.1993.tb13840.x. PMID 8242225.
- ↑ "Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen". The Cochrane Database of Systematic Reviews 12 (2): CD007245. December 2020. doi:10.1002/14651858.CD007245.pub4. PMID 33348436.
- ↑ "Breast cancer in men". CancerHelp UK. Cancer Research UK. 28 September 2007. http://www.cancerhelp.org.uk/help/default.asp?page=5075.
- ↑ Center for Drug Evaluation and Research (7 July 2005). "Tamoxifen Information: reducing the incidence of breast cancer in women at high risk". U.S. Food and Drug Administration. https://www.fda.gov/cder/news/tamoxifen/.
- ↑ "Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update". Journal of Clinical Oncology 32 (21): 2255–2269. July 2014. doi:10.1200/JCO.2013.54.2258. PMID 24868023.
- ↑ National Cancer Institute (26 April 2006). "Study of Tamoxifen and Raloxifene (STAR) Trial". U.S. National Institutes of Health. http://www.cancer.gov/star.
- ↑ University of Pittsburgh. "STAR Study of Tamoxifen and Raloxifen". http://www.nsabp.pitt.edu/STAR/Index.html.
- ↑ "Study Finds New Use for Raloxifene: Reducing Breast Cancer in High-Risk Postmenopausal Women". 22 April 2006. http://www.dslrf.org/breastcancer/content.asp?L2=2&L3=6&SID=130&CID=391&PID=14&CATID=0.
- ↑ "Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis". Human Reproduction 20 (6): 1511–1515. June 2005. doi:10.1093/humrep/deh840. PMID 15845599.
- ↑ "Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis". Andrology 1 (5): 749–757. September 2013. doi:10.1111/j.2047-2927.2013.00107.x. PMID 23970453.
- ↑ "Tamoxifen therapy for the management of pubertal gynecomastia: a systematic review". Journal of Pediatric Endocrinology & Metabolism 26 (9–10): 803–807. 2013. doi:10.1515/jpem-2013-0052. PMID 23729603.
- ↑ "Prevention of gynecomastia and breast pain caused by androgen deprivation therapy in prostate cancer: tamoxifen or radiotherapy?". International Journal of Radiation Oncology, Biology, Physics 83 (4): e519–e524. July 2012. doi:10.1016/j.ijrobp.2012.01.036. PMID 22704706.
- ↑ 35.0 35.1 "Treatment of Girls and Boys with McCune-Albright Syndrome with Precocious Puberty - Update 2017". Pediatric Endocrinology Reviews 15 (2): 136–141. December 2017. doi:10.17458/per.vol15.2017.nau.treatmentgirlsboys. PMID 29292624.
- ↑ 36.0 36.1 "Peripheral precocious puberty including congenital adrenal hyperplasia: causes, consequences, management and outcomes". Best Practice & Research. Clinical Endocrinology & Metabolism 33 (3): 101273. June 2019. doi:10.1016/j.beem.2019.04.007. PMID 31027974.
- ↑ "Disorders of Puberty: Pharmacotherapeutic Strategies for Management". Pediatric Pharmacotherapy. Handbook of Experimental Pharmacology. 261. May 2019. pp. 507–538. doi:10.1007/164_2019_208. ISBN 978-3-030-50493-9.
- ↑ Product Information: tamoxifen citrate oral tablets, tamoxifen citrate oral tablets. Watson Laboratories (per manufacturer), Corona, CA, 2011.
- ↑ Product Information: SOLTAMOX(R) oral solution, tamoxifen citrate oral solution. Midatech Pharma US Inc (per FDA), Raleigh, NC, 2018.
- ↑ OncoGenetics.Org (September 2009). "Medications Effective in Reducing Risk of Breast Cancer But Increase Risk of Adverse Effects". OncoGenetics.Org. http://www.oncogenetics.org/web/Medications-Effective-in-Reducing-Risk-of-Breast-Cancer-But-Increase-Risk-of-Adverse-Effects.
- ↑ "Antagonistic and agonistic effects of tamoxifen: significance in human cancer". Seminars in Oncology 24 (1 Suppl 1): S1-71-S1-80. February 1997. PMID 9045319.
- ↑ "Tamoxifen (TAM): the dispute goes on". Annali dell'Istituto Superiore di Sanità 42 (2): 170–173. 2006. PMID 17033137. http://www.iss.it/publ/anna/2006/2/422170.pdf. Retrieved 3 July 2007.
- ↑ "Tamoxifen for Breast Cancer & Side Effects". Health and Life. 11 December 2009. http://healthlifeandstuff.com/2009/12/tamoxifen-for-breast-cancer-side-effects/.
- ↑ "Known and Probable Carcinogens". American Cancer Society. 3 February 2006. http://www.cancer.org/docroot/PED/content/PED_1_3x_Known_and_Probable_Carcinogens.asp?sitearea=PED.
- ↑ "Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women". Breast 15 (3): 301–312. June 2006. doi:10.1016/j.breast.2005.08.033. PMID 16230014.
- ↑ "Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial". Circulation 111 (5): 650–656. February 2005. doi:10.1161/01.CIR.0000154545.84124.AC. PMID 15699284.
- ↑ "Toremifene: an evaluation of its safety profile". Breast 15 (2): 142–157. April 2006. doi:10.1016/j.breast.2005.09.007. PMID 16289904.
- ↑ 48.0 48.1 Drug-Induced Hepatotoxicity. Springer Science & Business Media. 6 December 2012. pp. 565–. ISBN 978-3-642-61013-4. https://books.google.com/books?id=xZf-CAAAQBAJ&pg=PA565.
- ↑ "Tamoxifen-induced non-alcoholic steatohepatitis: where are we now and where are we going?". Expert Opinion on Drug Safety 6 (1): 1–4. January 2007. doi:10.1517/14740338.6.1.1. PMID 17181445.
- ↑ "Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes". Journal of Clinical Oncology 23 (36): 9312–9318. December 2005. doi:10.1200/JCO.2005.03.3266. PMID 16361630.
- ↑ "CYP2D6 polymorphisms and the impact on tamoxifen therapy". Journal of Pharmaceutical Sciences 96 (9): 2224–2231. September 2007. doi:10.1002/jps.20892. PMID 17518364.
- ↑ Information about CYP2D6 and tamoxifen from DNADirect's website
- ↑ "Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen". JAMA 302 (13): 1429–1436. October 2009. doi:10.1001/jama.2009.1420. PMID 19809024.
- ↑ Information about Tamoxitest and how DNA testing can help in the selection of the best treatment methodology from Genelex's website
- ↑ "Tamoxifen in early-stage estrogen receptor-positive breast cancer: overview of clinical use and molecular biomarkers for patient selection". OncoTargets and Therapy 4: 1–11. December 2010. doi:10.2147/OTT.S10155. PMID 21552410.
- ↑ "CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment". Journal of the National Cancer Institute 97 (1): 30–39. January 2005. doi:10.1093/jnci/dji005. PMID 15632378.
- ↑ Staff Reports (Summer 2009). "ASCO Updates: Antidepressants Reduce the Effectiveness of Tamoxifen.". CURE (Cancer Updates, Research and Education). http://www.curetoday.com/index.cfm/fuseaction/article.show/id/2/article_id/1152.
- ↑ "Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study". BMJ 340: c693. February 2010. doi:10.1136/bmj.c693. PMID 20142325.
- ↑ PDB: 3ERT; "The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen". Cell 95 (7): 927–937. December 1998. doi:10.1016/S0092-8674(00)81717-1. PMID 9875847.
- ↑ "Identification of estrogen-responsive genes by complementary deoxyribonucleic acid microarray and characterization of a novel early estrogen-induced gene: EEIG1". Molecular Endocrinology 18 (2): 402–411. February 2004. doi:10.1210/me.2003-0202. PMID 14605097.
- ↑ "Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts". Cell 130 (5): 811–823. September 2007. doi:10.1016/j.cell.2007.07.025. PMID 17803905.
- ↑ "Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival". The EMBO Journal 27 (3): 535–545. February 2008. doi:10.1038/sj.emboj.7601984. PMID 18219273.
- ↑ "Prevention and treatment of osteoporosis in women with breast cancer". Mayo Clinic Proceedings 75 (8): 821–829. August 2000. doi:10.4065/75.8.821. PMID 10943237.
- ↑ Gynäkologische Endokrinologie und Fortpflanzungsmedizin: Band 1: Gynäkologische Endokrinologie. Springer-Verlag. 17 April 2013. pp. 88–. ISBN 978-3-662-07635-4. https://books.google.com/books?id=mBF9BwAAQBAJ&pg=PA88.
- ↑ "Current status of estrogen therapy for the menopause". Fertility and Sterility 37 (1): 5–25. January 1982. doi:10.1016/S0015-0282(16)45970-4. PMID 6277697.
- ↑ "Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects". Clinical Pharmacology and Therapeutics 88 (6): 814–817. December 2010. doi:10.1038/clpt.2010.196. PMID 20981001.
- ↑ "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ "Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist". Obstetrics and Gynecology 105 (5 Pt 1): 953–961. May 2005. doi:10.1097/01.AOG.0000161958.70059.db. PMID 15863530.
- ↑ "Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping". Cancer Treatment Reviews 41 (3): 289–299. March 2015. doi:10.1016/j.ctrv.2015.01.002. PMID 25618289.
- ↑ "Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription". Cell 103 (6): 843–852. December 2000. doi:10.1016/S0092-8674(00)00188-4. PMID 11136970.
- ↑ "Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function". Cancer Research 68 (3): 826–833. February 2008. doi:10.1158/0008-5472.CAN-07-2707. PMID 18245484.
- ↑ 72.0 72.1 72.2 "Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen". Nature 456 (7222): 663–666. December 2008. doi:10.1038/nature07483. PMID 19005469. Bibcode: 2008Natur.456..663H.
- ↑ "Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer". Journal of the National Cancer Institute 95 (5): 353–361. March 2003. doi:10.1093/jnci/95.5.353. PMID 12618500.
- ↑ "New Mechanism Predicts Tamoxifen Response: PAX2 gene implicated in tamoxifen-induced inhibition of ERBB2/HER2-mediated tumor growth". www.modernmedicine.com. 13 November 2008. http://www.modernmedicine.com/modernmedicine/Modern+Medicine+Now/New-Mechanism-Predicts-Tamoxifen-Response/ArticleNewsFeed/Article/detail/565990.
- ↑ "Study sheds new light on tamoxifen resistance". News. CORDIS News. http://cordis.europa.eu/fetch?CALLER=EN_NEWS&ACTION=D&SESSION=&RCN=30093.
- ↑ 76.0 76.1 76.2 "Hormone-related pharmacokinetic variations associated with anti-breast cancer drugs". Expert Opinion on Drug Metabolism & Toxicology 9 (9): 1085–1095. September 2013. doi:10.1517/17425255.2013.802771. PMID 23687971.
- ↑ "Selective estrogen-receptor modulators for primary prevention of breast cancer". Journal of Clinical Oncology 23 (8): 1644–1655. March 2005. doi:10.1200/JCO.2005.11.005. PMID 15755972.
- ↑ 78.0 78.1 "Estrogens and selective estrogen receptor modulators in acromegaly". Endocrine 54 (2): 306–314. November 2016. doi:10.1007/s12020-016-1118-z. PMID 27704479.
- ↑ 79.0 79.1 "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacological Reviews 67 (3): 505–540. July 2015. doi:10.1124/pr.114.009712. PMID 26023144.
- ↑ "Estrogen-related receptors as emerging targets in cancer and metabolic disorders". Current Topics in Medicinal Chemistry 6 (3): 203–215. 2006. doi:10.2174/1568026610606030203. PMID 16515477.
- ↑ "Inhibition of cytochrome p450 enzymes by the e- and z-isomers of norendoxifen". Drug Metabolism and Disposition 41 (9): 1715–1720. September 2013. doi:10.1124/dmd.113.052506. PMID 23824607.
- ↑ 82.0 82.1 "Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder". CNS Drugs 23 (7): 569–582. 2009. doi:10.2165/00023210-200923070-00003. PMID 19552485.
- ↑ 83.0 83.1 83.2 Prostate and Other Genitourinary Cancers: From Cancer: Principles & Practice of Oncology, 10th edition. Wolters Kluwer Health. 18 March 2016. pp. 990–. ISBN 978-1-4963-5421-1. https://books.google.com/books?id=Bf3DCwAAQBAJ&pg=PT990.
- ↑ 84.0 84.1 "Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial". Clinical Cancer Research 10 (7): 2336–2343. April 2004. doi:10.1158/1078-0432.ccr-03-0538. PMID 15073109.
- ↑ "Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance". Breast Cancer Research and Treatment 2 (2): 123–138. 1982. doi:10.1007/BF01806449. PMID 6184101.
- ↑ "Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment". Cancer Research 49 (8): 2175–2183. April 1989. PMID 2702659. https://cancerres.aacrjournals.org/content/49/8/2175.short.
- ↑ "Pharmacogenomics of tamoxifen therapy". Clinical Chemistry 55 (10): 1770–1782. October 2009. doi:10.1373/clinchem.2008.121756. PMID 19574470.
- ↑ 88.0 88.1 Estrogen/antiestrogen Action and Breast Cancer Therapy. Univ of Wisconsin Press. 1986. pp. 28,154. ISBN 978-0-299-10480-1. https://books.google.com/books?id=7WmLZfGXST0C&pg=PA28.
- ↑ The Anticancer Drugs. Oxford University Press. 1994. pp. 21–. ISBN 978-0-19-506739-2. https://books.google.com/books?id=nPR1L4K5HuEC&pg=PA21.
- ↑ 90.0 90.1 Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. 23 July 2013. pp. 7–. ISBN 978-3-0348-0664-0. https://books.google.com/books?id=p-W5BAAAQBAJ&pg=PA7.
- ↑ 91.0 91.1 The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. 11 January 2011. pp. 177–178. ISBN 978-3-527-32669-3. https://books.google.com/books?id=iDNy0XxGqT8C&pg=PA177.
- ↑ Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs. Springer Science & Business Media. 22 September 2006. pp. 52–. ISBN 978-3-540-34742-2. https://books.google.com/books?id=heJDAAAAQBAJ&pg=PA52.
- ↑ 93.0 93.1 Osteoporosis in Men: The Effects of Gender on Skeletal Health. Academic Press. 30 November 2009. pp. 717–. ISBN 978-0-08-092346-8. https://books.google.com/books?id=nfWNYFdOsCsC&pg=PA717.
- ↑ Drug Discovery: A History. New York: Wiley. 2005. p. 472 pages. ISBN 978-0-471-89979-2.
- ↑ 95.0 95.1 "Tamoxifen: a most unlikely pioneering medicine". Nature Reviews. Drug Discovery 2 (3): 205–213. March 2003. doi:10.1038/nrd1031. PMID 12612646.
- ↑ "Dog's tale of survival opens door in cancer research". Health and Behavior. USA Today. 24 July 2002. https://www.usatoday.com/news/health/2002-07-24-cover-cancer_x.htm.
- ↑ "Tamoxifen inhibits angiogenesis in estrogen receptor-negative animal models". Clinical Cancer Research 6 (11): 4359–4364. November 2000. PMID 11106254. http://clincancerres.aacrjournals.org/cgi/content/abstract/6/11/4359.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid12796359 - ↑ "Adjuvant Antihormone Therapy". Estrogen Action, Selective Estrogen Receptor Modulators And Women's Health: Progress And Promise. World Scientific. 2013. pp. 229–254. doi:10.1142/9781848169586_0010. ISBN 978-1-84816-959-3. https://books.google.com/books?id=ZM26CgAAQBAJ&pg=PA229.
- ↑ "A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474". British Journal of Cancer 25 (2): 270–275. June 1971. doi:10.1038/bjc.1971.33. PMID 5115829.
- ↑ "Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels". British Medical Journal 1 (5844): 13–14. January 1973. doi:10.1136/bmj.1.5844.13. PMID 4567104.
- ↑ "Maverick and pioneer whose work is improving odds in breast cancer fight". http://www.yorkshirepost.co.uk/news/features/maverick-and-pioneer-whose-work-is-improving-odds-in-breast-cancer-fight-1-5799838.
- ↑ "Maverick and pioneer whose work is improving odds in breast cancer fight". The Yorkshire Post. 27 June 2013. http://www.yorkshirepost.co.uk/news/analysis/maverick-and-pioneer-whose-work-is-improving-odds-in-breast-cancer-fight-1-5799838.
- ↑ "Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer". Lancet 2 (8347): 450. August 1983. doi:10.1016/S0140-6736(83)90406-3. PMID 6135926.
- ↑ "The pharmacology and clinical uses of tamoxifen". Pharmacology & Therapeutics 25 (2): 127–205. 1984. doi:10.1016/0163-7258(84)90043-3. PMID 6438654.
- ↑ "Selective estrogen receptor modulation: a personal perspective". Cancer Research 61 (15): 5683–5687. August 2001. PMID 11479197.
- ↑ "Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture". Cancer Research 50 (14): 4177–4189. July 1990. PMID 2194650.
- ↑ Early Breast Cancer Trialists' Collaborative Group (May 1998). "Tamoxifen for early breast cancer: an overview of the randomised trials.". Lancet 351 (9114): 1451–1467. doi:10.1016/S0140-6736(97)11423-4. PMID 9605801.
- ↑ "Tamoxifen". https://www.drugs.com/international/tamoxifen.html.
- ↑ "Cancer the generic impact". BioPortfolio Limited. http://www.bioportfolio.com/news/datamonitor_16.htm.
- ↑ "AstraZenecain Cancer: Slide #15". AstraZeneca Annual Business Review. www.astrazeneca.com. http://www.astrazeneca.com/_mshost3690701/content/resources/media/investors/2004-abr-brent-vose-oncology.pdf. "2004 tamoxifen market share: 70% Source: IMS HEALTH, IMS MIDAS Monthly. July 2004. Aromatase Inhibitors + Tamoxifen"
- ↑ "Tamoxifen treatment of progressive precocious puberty in a patient with McCune-Albright syndrome". Journal of Pediatric Endocrinology & Metabolism 12 (5): 681–686. 1999. doi:10.1515/jpem.1999.12.5.681. PMID 10703542.
- ↑ "Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial". The Journal of Pediatrics 143 (1): 60–66. July 2003. doi:10.1016/S0022-3476(03)00128-8. PMID 12915825.
- ↑ "The use of tamoxifen to improve height potential in short pubertal boys". Pediatrics 116 (6): 1513–1515. December 2005. doi:10.1542/peds.2005-0577. PMID 16322179.
- ↑ "Tamoxifen impairs both longitudinal and cortical bone growth in young male rats". Journal of Bone and Mineral Research 23 (8): 1267–1277. August 2008. doi:10.1359/jbmr.080319. PMID 18348701.
- ↑ "Tamoxifen induces permanent growth arrest through selective induction of apoptosis in growth plate chondrocytes in cultured rat metatarsal bones". Bone 40 (5): 1415–1424. May 2007. doi:10.1016/j.bone.2006.12.066. PMID 17293177.
- ↑ "Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis". Annals of Internal Medicine 144 (2): 101–106. January 2006. doi:10.7326/0003-4819-144-2-200601170-00007. PMID 16418409.
- ↑ "Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients". Clinical Gastroenterology and Hepatology 5 (5): 589–96; quiz 523–4. May 2007. doi:10.1016/j.cgh.2007.02.032. PMID 17478346.
- ↑ "Riedel's thyroiditis treated with tamoxifen". Croatian Medical Journal 44 (2): 239–241. April 2003. PMID 12698518. http://www.cmj.hr/2003/44/2/12698518.pdf.
- ↑ "Ligand-activated site-specific recombination in mice". Proceedings of the National Academy of Sciences of the United States of America 93 (20): 10887–10890. October 1996. doi:10.1073/pnas.93.20.10887. PMID 8855277. Bibcode: 1996PNAS...9310887F.
- ↑ 121.0 121.1 121.2 "Tamoxifen: A Protein Kinase C Inhibitor to Treat Mania: A Systematic Review and Meta-Analysis of Randomized, Placebo-Controlled Trials". Journal of Clinical Psychopharmacology 36 (3): 272–275. June 2016. doi:10.1097/JCP.0000000000000492. PMID 27088436.
- ↑ 122.0 122.1 "Role of Protein Kinase C in Bipolar Disorder: A Review of the Current Literature". Molecular Neuropsychiatry 3 (2): 108–124. November 2017. doi:10.1159/000480349. PMID 29230399.
- ↑ "Endoxifen - Intas Pharmaceuticals/Jina pharmaceuticals". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800036114.
- ↑ "Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives". European Journal of Medicinal Chemistry 143: 515–531. January 2018. doi:10.1016/j.ejmech.2017.11.056. PMID 29207335.
Further reading
- "Tamoxifen Therapy and CYP2D6 Genotype". Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). 2014. Bookshelf ID: NBK247013. https://www.ncbi.nlm.nih.gov/books/NBK247013/.
External links
- "Tamoxifen". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tamoxifen.
- "Tamoxifen citrate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tamoxifen%20citrate.
- "Tamoxifen citrate". 5 October 2006. https://www.cancer.gov/about-cancer/treatment/drugs/tamoxifencitrate.
 |