Chemistry:Cannabinoid
Cannabinoids (/kəˈnæbənɔɪdzˌ ˈkænəbənɔɪdz/) are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds.[1][2] The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis.[3][4] Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties.[5] At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin.[6] It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort,[7] and earlier in Echinacea.
Phytocannabinoids are multi-ring phenolic compounds structurally related to THC,[8] but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include aminoalkylindoles, 1,5-diarylpyrazoles, quinolines, and arylsulfonamides as well as eicosanoids related to endocannabinoids.[3]
Uses
Medical uses include the treatment of nausea due to chemotherapy, spasticity, and possibly neuropathic pain.[9] Common side effects include dizziness, sedation, confusion, dissociation, and "feeling high".[9]
Cannabinoid receptors
Before the 1980s, cannabinoids were speculated to produce their physiological and behavioral effects via nonspecific interaction with cell membranes, instead of interacting with specific membrane-bound receptors. The discovery of the first cannabinoid receptors in the 1980s helped to resolve this debate.[10] These receptors are common in animals. Two known cannabinoid receptors are termed CB1 and CB2,[11] with mounting evidence of more.[12] The human brain has more cannabinoid receptors than any other G protein-coupled receptor (GPCR) type.[13]
The Endocannabinoid System (ECS) regulates many functions of the human body. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.[14]
Cannabinoid receptor type 1
CB1 receptors are found primarily in the brain, more specifically in the basal ganglia and in the limbic system, including the hippocampus[11] and the striatum. They are also found in the cerebellum and in both male and female reproductive systems. CB1 receptors are absent in the medulla oblongata, the part of the brain stem responsible for respiratory and cardiovascular functions. CB1 is also found in the human anterior eye and retina.[15]
Cannabinoid receptor type 2
CB2 receptors are predominantly found in the immune system, or immune-derived cells[16][17][18][19] with varying expression patterns. While found only in the peripheral nervous system, a report does indicate that CB2 is expressed by a subpopulation of microglia in the human cerebellum.[20] CB2 receptors appear to be responsible for immunomodulatory[19] and possibly other therapeutic effects of cannabinoid as seen in vitro and in animal models.[18]
Phytocannabinoids


The classical cannabinoids are concentrated in a viscous resin produced in structures known as glandular trichomes. At least 113 different cannabinoids have been isolated from the Cannabis plant.[6]
All classes derive from cannabigerol-type (CBG) compounds and differ mainly in the way this precursor is cyclized.[21] The classical cannabinoids are derived from their respective 2-carboxylic acids (2-COOH) by decarboxylation (catalyzed by heat, light, or alkaline conditions).[22]
Well known cannabinoids
The best studied cannabinoids include tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN).
Tetrahydrocannabinol
Tetrahydrocannabinol (THC) is the primary psychoactive component of the Cannabis plant. Delta-9-tetrahydrocannabinol (Δ9-THC, THC) and delta-8-tetrahydrocannabinol (Δ8-THC), through intracellular CB1 activation, induce anandamide and 2-arachidonoylglycerol synthesis produced naturally in the body and brain[citation needed][dubious ]. These cannabinoids produce the effects associated with cannabis by binding to the CB1 cannabinoid receptors in the brain.[23]
Cannabidiol
Cannabidiol (CBD) is mildly psychotropic. Evidence shows that the compound counteracts cognitive impairment associated with the use of cannabis.[24] Cannabidiol has little affinity for CB1 and CB2 receptors but acts as an indirect antagonist of cannabinoid agonists.[25] It was found to be an antagonist at the putative new cannabinoid receptor, GPR55, a GPCR expressed in the caudate nucleus and putamen.[26] Cannabidiol has also been shown to act as a 5-HT1A receptor agonist.[27] CBD can interfere with the uptake of adenosine, which plays an important role in biochemical processes, such as energy transfer. It may play a role in promoting sleep and suppressing arousal.[28]
CBD shares a precursor with THC and is the main cannabinoid in CBD-dominant Cannabis strains. CBD has been shown to play a role in preventing the short-term memory loss associated with THC.[29]
There is tentative evidence that CBD has an anti-psychotic effect, but research in this area is limited.[30][24]
Cannabinol
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors.[31][32][33] Through its mechanism of partial agonism at the CB1R, CBN is thought to interact with other kinds of neurotransmission (e.g., dopaminergic, serotonergic, cholinergic, and noradrenergic).
CBN was the first cannabis compound to be isolated from cannabis extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940[34], followed by some of the first pre-clinical research studies to determine the effects of individual cannabis-derived compounds in vivo.[35] Although CBN shares the same mechanism of action as other more well-known phytocannabinoids (e.g., delta-9 tetrahydrocannabinol or D9THC), it has a lower affinity for CB1 receptors, meaning that much higher doses of CBN are required in order to experience physiologic effects (e.g., mild sedation) associated with CB1R agonism.[36][35] Although scientific reports are conflicting, the majority of findings suggest that CBN has a slightly higher affinity for CB2 as compared to CB1. Although CBN has been marketed as a sleep aid in recent years, there is a lack of scientific evidence to support these claims, warranting skepticism on the part of consumers.[36]
Biosynthesis
Cannabinoid production starts when an enzyme causes geranyl pyrophosphate and olivetolic acid to combine and form CBGA. Next, CBGA is independently converted to either CBG, THCA, CBDA or CBCA by four separate synthase, FAD-dependent dehydrogenase enzymes. There is no evidence for enzymatic conversion of CBDA or CBD to THCA or THC. For the propyl homologues (THCVA, CBDVA and CBCVA), there is an analogous pathway that is based on CBGVA from divarinolic acid instead of olivetolic acid.
Double bond position
In addition, each of the compounds above may be in different forms depending on the position of the double bond in the alicyclic carbon ring. There is potential for confusion because there are different numbering systems used to describe the position of this double bond. Under the dibenzopyran numbering system widely used today, the major form of THC is called Δ9-THC, while the minor form is called Δ8-THC. Under the alternate terpene numbering system, these same compounds are called Δ1-THC and Δ6-THC, respectively.
Length
Most classical cannabinoids are 21-carbon compounds. However, some do not follow this rule, primarily because of variation in the length of the side-chain attached to the aromatic ring. In THC, CBD, and CBN, this side-chain is a pentyl (5-carbon) chain. In the most common homologue, the pentyl chain is replaced with a propyl (3-carbon) chain. Cannabinoids with the propyl side chain are named using the suffix varin and are designated THCV, CBDV, or CBNV, while those with the heptyl side chain are named using the suffix phorol and are designated THCP and CBDP.
Cannabinoids in other plants
Phytocannabinoids are known to occur in several plant species besides cannabis. These include Echinacea purpurea, Echinacea angustifolia, Acmella oleracea, Helichrysum umbraculigerum, and Radula marginata.[37] The best-known cannabinoids that are not derived from Cannabis are the lipophilic alkamides (alkylamides) from Echinacea species, most notably the cis/trans isomers dodeca-2E,4E,8Z,10E/Z-tetraenoic-acid-isobutylamide.[37] At least 25 different alkylamides have been identified, and some of them have shown affinities to the CB2-receptor.[38][39] In some Echinacea species, cannabinoids are found throughout the plant structure, but are most concentrated in the roots and flowers.[40][41] Yangonin found in the Kava plant has significant affinity to the CB1 receptor.[42] Tea (Camellia sinensis) catechins have an affinity for human cannabinoid receptors.[43] A widespread dietary terpene, beta-caryophyllene, a component from the essential oil of cannabis and other medicinal plants, has also been identified as a selective agonist of peripheral CB2-receptors, in vivo.[44] Black truffles contain anandamide.[45] Perrottetinene, a moderately psychoactive cannabinoid,[46] has been isolated from different Radula varieties. Machaeriol A and related compounds are found in plants from the Machaerium family.[47]
Most of the phytocannabinoids are nearly insoluble in water but are soluble in lipids, alcohols, and other non-polar organic solvents.
Cannabis plant profile
Cannabis plants can exhibit wide variation in the quantity and type of cannabinoids they produce. The mixture of cannabinoids produced by a plant is known as the plant's cannabinoid profile. Selective breeding has been used to control the genetics of plants and modify the cannabinoid profile. For example, strains that are used as fiber (commonly called hemp) are bred such that they are low in psychoactive chemicals like THC. Strains used in medicine are often bred for high CBD content, and strains used for recreational purposes are usually bred for high THC content or for a specific chemical balance.
Quantitative analysis of a plant's cannabinoid profile is often determined by gas chromatography (GC), or more reliably by gas chromatography combined with mass spectrometry (GC/MS). Liquid chromatography (LC) techniques are also possible and, unlike GC methods, can differentiate between the acid and neutral forms of the cannabinoids. There have been systematic attempts to monitor the cannabinoid profile of cannabis over time, but their accuracy is impeded by the illegal status of the plant in many countries.
Pharmacology
Cannabinoids can be administered by smoking, vaporizing, oral ingestion, transdermal patch, intravenous injection, sublingual absorption, or rectal suppository. Once in the body, most cannabinoids are metabolized in the liver, especially by cytochrome P450 mixed-function oxidases, mainly CYP 2C9.[48] Thus supplementing with CYP 2C9 inhibitors leads to extended intoxication.[48]
Some is also stored in fat in addition to being metabolized in the liver. Δ9-THC is metabolized to 11-hydroxy-Δ9-THC, which is then metabolized to 9-carboxy-THC.[49] Some cannabis metabolites can be detected in the body several weeks after administration. These metabolites are the chemicals recognized by common antibody-based "drug tests"; in the case of THC or others, these loads do not represent intoxication (compare to ethanol breath tests that measure instantaneous blood alcohol levels), but an integration of past consumption over an approximately month-long window. This is because they are fat-soluble, lipophilic molecules that accumulate in fatty tissues.[50]
Research shows the effect of cannabinoids might be modulated by aromatic compounds produced by the cannabis plant, called terpenes. This interaction would lead to the entourage effect.[51]
Modulation of mitochondrial activity
Recent evidence has shown that cannabinoids play a role in the modulation of various mitochondrial processes, including intracellular calcium regulation, activation of apoptosis, impairment of electron transport chain activity, disruption of mitochondrial respiration and ATP production, and regulation of mitochondrial dynamics. These processes contribute to various aspects of cellular biology and can be modified in response to external stimuli. The interaction between cannabinoids and mitochondria is complex, and various molecular mechanisms have been proposed, including direct effects on mitochondrial membranes and receptor-mediated effects. However, an integrated hypothesis of cannabinoids' actions on these processes has yet to be formulated due to conflicting data and the complexity of the pathways involved.[52]
Cannabinoid-based pharmaceuticals
Nabiximols (brand name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC.[53] Also included are minor cannabinoids and terpenoids, ethanol and propylene glycol excipients, and peppermint flavoring.[54] The drug, made by GW Pharmaceuticals, was first approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis, making it the first cannabis-based medicine. It is marketed by Bayer in Canada.[55] Sativex has been approved in 25 countries; clinical trials are underway in the United States to gain FDA approval.[56] In 2007, it was approved for treatment of cancer pain.[54] In Phase III trials, the most common adverse effects were dizziness, drowsiness and disorientation; 12% of subjects stopped taking the drug because of the side effects.[57]
Dronabinol (brand names Marinol and Syndros) is a delta-9-THC containing drug for treating HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting.[58]
The CBD drug Epidiolex has been approved by the Food and Drug Administration for treatment of two rare and severe forms of epilepsy,[59] Dravet and Lennox-Gastaut syndromes.[60]
Nabilone (Cesamet) is an FDA approved synthetic analog of THC, prescribed for the treatment of nausea and vomiting induced by chemotherapy treatment in people who have failed to respond adequately to conventional antiemetic treatments.[58]
Separation
Cannabinoids can be separated from the plant by extraction with organic solvents. Hydrocarbons and alcohols are often used as solvents. However, these solvents are flammable and many are toxic.[61] Butane may be used, which evaporates extremely quickly. Supercritical solvent extraction with carbon dioxide is an alternative technique. Once extracted, isolated components can be separated using wiped film vacuum distillation or other distillation techniques.[62] Also, techniques such as SPE or SPME are found useful in the extraction of these compounds.[63]
History
The first discovery of an individual cannabinoid was made, when British chemist Robert S. Cahn reported the partial structure of Cannabinol (CBN), which he later identified as fully formed in 1940.
Two years later, in 1942,[64] American chemist, Roger Adams, made history when he discovered Cannabidiol (CBD).[65] Progressing from Adams research, in 1963[66] Israeli professor Raphael Mechoulam[67] later identified the stereochemistry of CBD. The following year, in 1964,[66] Mechoulam and his team identified the stereochemistry of Tetrahydrocannabinol (THC).[citation needed]
Due to molecular similarity and ease of synthetic conversion, CBD was originally believed to be a natural precursor to THC. However, it is now known that CBD and THC are produced independently in the Cannabis plant from the precursor CBG.[citation needed]
Emergence of derived psychoactive cannabis products
The Agriculture Improvement Act of 2018 has been interpreted as allowing any hemp-derived product not exceeding 0.3% Δ9-THC to be sold legally in the US. Because the law limited only Δ9-THC levels, many other cannabinoids are generally considered legal to sell and are widely available in stores and online, including Δ8-THC, Δ10-THC, HHC, and THCP,[68][69] but have not had the same in-depth research that the Δ9 isomer has on the human body; carrying potential risks in the short- or long-term. Other concerns include difficulties for drug testing due to novel metabolites, or high potency/binding affinity of isomers for cannabinoid receptors showing potential for abuse (i.e., THCP, which has 33× the binding affinity of Δ9-THC)[70][71] From 2021 to 2023, the Δ8-THC market generated US$2 billion in revenue.[72] Many substances are scheduled at the state level under various synonyms owing to the different dibenzopyran and monoterpenoid naming conventions. Delta-1, Delta-6, and Delta 3,4-Tetrahydrocannabinol are alternative names for Delta-9, Delta-8, and Delta-6a10a Tetrahydrocannabinol, respectively.[73]
A 2023 paper seeking the regulation of cannabinoid terminology coined the term "derived psychoactive cannabis products" to accurately and usefully distinguish said products whilst excluding unrelated substances.[74]
Endocannabinoids are substances produced from within the body that activate cannabinoid receptors. After the discovery of the first cannabinoid receptor in 1988, scientists began searching for endogenous ligand for the receptors.[10][75]
Types of endocannabinoid ligands
Arachidonoylethanolamine (Anandamide or AEA)
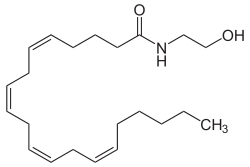
Anandamide was the first such compound identified as arachidonoyl ethanolamine. The name is derived from ananda, the Sanskrit word for bliss. It has a pharmacology similar to THC, although its structure is quite different. Anandamide binds to the central (CB1) and, to a lesser extent, peripheral (CB2) cannabinoid receptors, where it acts as a partial agonist. Anandamide is about as potent as THC at the CB1 receptor.[76] Anandamide is found in nearly all tissues in a wide range of animals.[77] Anandamide has also been found in plants, including small amounts in chocolate.[78]
Two analogs of anandamide, 7,10,13,16-docosatetraenoylethanolamide and homo-γ-linolenoylethanolamine, have similar pharmacology. All of these compounds are members of a family of signalling lipids called N-acylethanolamines, which also includes the noncannabimimetic palmitoylethanolamide and oleoylethanolamide, which possess anti-inflammatory and anorexigenic effects, respectively. Many N-acylethanolamines have also been identified in plant seeds[79] and in molluscs.[80]
2-Arachidonoylglycerol (2-AG)
Another endocannabinoid, 2-arachidonoylglycerol, binds to both the CB1 and CB2 receptors with similar affinity, acting as a full agonist at both.[76] 2-AG is present at significantly higher concentrations in the brain than anandamide,[81] and there is some controversy over whether 2-AG rather than anandamide is chiefly responsible for endocannabinoid signalling in vivo.[11] In particular, one in vitro study suggests that 2-AG is capable of stimulating higher G-protein activation than anandamide, although the physiological implications of this finding are not yet known.[82]
2-Arachidonyl glyceryl ether (noladin ether)
In 2001, a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (noladin ether), was isolated from porcine brain.[83] Prior to this discovery, it had been synthesized as a stable analog of 2-AG; indeed, some controversy remains over its classification as an endocannabinoid, as another group failed to detect the substance at "any appreciable amount" in the brains of several different mammalian species.[84] It binds to the CB1 cannabinoid receptor (Ki = 21.2 nmol/L) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds primarily to the CB1 receptor, and only weakly to the CB2 receptor.[76]
N-Arachidonoyl dopamine (NADA)
Discovered in 2000, NADA preferentially binds to the CB1 receptor.[85] Like anandamide, NADA is also an agonist for the vanilloid receptor subtype 1 (TRPV1), a member of the vanilloid receptor family.[86][87]
Virodhamine (OAE)
A fifth endocannabinoid, virodhamine, or O-arachidonoyl-ethanolamine (OAE), was discovered in June 2002. Although it is a full agonist at CB2 and a partial agonist at CB1, it behaves as a CB1 antagonist in vivo. In rats, virodhamine was found to be present at comparable or slightly lower concentrations than anandamide in the brain, but 2- to 9-fold higher concentrations peripherally.[88]
Lysophosphatidylinositol (LPI)
Lysophosphatidylinositol is the endogenous ligand to novel endocannabinoid receptor GPR55, making it a strong contender as the sixth endocannabinoid.[89]
Function
Endocannabinoids serve as intercellular 'lipid messengers',[90] signaling molecules that are released from one cell and activating the cannabinoid receptors present on other nearby cells. Although in this intercellular signaling role they are similar to the well-known monoamine neurotransmitters such as dopamine, endocannabinoids differ in numerous ways from them. For instance, they are used in retrograde signaling between neurons.[91] Furthermore, endocannabinoids are lipophilic molecules that are not very soluble in water. They are not stored in vesicles and exist as integral constituents of the membrane bilayers that make up cells. They are believed to be synthesized 'on-demand' rather than made and stored for later use.
As hydrophobic molecules, endocannabinoids cannot travel unaided for long distances in the aqueous medium surrounding the cells from which they are released and therefore act locally on nearby target cells. Hence, although emanating diffusely from their source cells, they have much more restricted spheres of influence than do hormones, which can affect cells throughout the body.
The mechanisms and enzymes underlying the biosynthesis of endocannabinoids remain elusive and continue to be an area of active research.
The endocannabinoid 2-AG has been found in bovine and human maternal milk.[92]
A review by Matties et al. (1994) summed up the phenomenon of gustatory enhancement by certain cannabinoids.[93] The sweet receptor (Tlc1) is stimulated by indirectly increasing its expression and suppressing the activity of leptin, the Tlc1 antagonist. It is proposed that the competition of leptin and cannabinoids for Tlc1 is implicated in energy homeostasis.[94]
Retrograde signal
Conventional neurotransmitters are released from a ‘presynaptic’ cell and activate appropriate receptors on a ‘postsynaptic’ cell, where presynaptic and postsynaptic designate the sending and receiving sides of a synapse, respectively. Endocannabinoids, on the other hand, are described as retrograde transmitters because they most commonly travel ‘backward’ against the usual synaptic transmitter flow. They are, in effect, released from the postsynaptic cell and act on the presynaptic cell, where the target receptors are densely concentrated on axonal terminals in the zones from which conventional neurotransmitters are released. Activation of cannabinoid receptors temporarily reduces the amount of conventional neurotransmitter released. This endocannabinoid-mediated system permits the postsynaptic cell to control its own incoming synaptic traffic. The ultimate effect on the endocannabinoid-releasing cell depends on the nature of the conventional transmitter being controlled. For instance, when the release of the inhibitory transmitter GABA is reduced, the net effect is an increase in the excitability of the endocannabinoid-releasing cell. On the converse, when release of the excitatory neurotransmitter glutamate is reduced, the net effect is a decrease in the excitability of the endocannabinoid-releasing cell.[95] [citation needed]
"Runner's high"
The runner's high, the feeling of euphoria that sometimes accompanies aerobic exercise, has often been attributed to the release of endorphins, but newer research suggests that it might be due to endocannabinoids instead.[96]
Synthetic cannabinoids
Historically, laboratory synthesis of cannabinoids was often based on the structure of herbal cannabinoids, and a large number of analogs have been produced and tested, especially in a group led by Roger Adams as early as 1941 and later in a group led by Raphael Mechoulam.[97] Newer compounds are no longer related to natural cannabinoids or are based on the structure of the endogenous cannabinoids.[98]
Synthetic cannabinoids are particularly useful in experiments to determine the relationship between the structure and activity of cannabinoid compounds, by making systematic, incremental modifications of cannabinoid molecules.[99]
When synthetic cannabinoids are used recreationally, they present significant health dangers to users.[100] In the period of 2012 through 2014, over 10,000 contacts to poison control centers in the United States were related to use of synthetic cannabinoids.[100]
Medications containing natural or synthetic cannabinoids or cannabinoid analogs:
- Dronabinol (Marinol), is synthetic Δ9-tetrahydrocannabinol (THC), used as an appetite stimulant, anti-emetic, and analgesic
- Nabilone (Cesamet, Canemes), a synthetic cannabinoid and an analog of Marinol. It is Schedule II unlike Marinol, which is Schedule III
- Rimonabant (SR141716), a selective cannabinoid (CB1) receptor inverse agonist once used as an anti-obesity drug under the proprietary name Acomplia. It was also used for smoking cessation
Other notable synthetic cannabinoids include:
- JWH-018, a potent synthetic cannabinoid agonist discovered by John W. Huffman at Clemson University. It was often sold in legal smoke blends collectively known as "spice". Several countries and states have moved to ban it legally.
- JWH-073
- CP-55940, produced in 1974, this synthetic cannabinoid receptor agonist is many times more potent than THC.
- Dimethylheptylpyran
- HU-210, about 100 times as potent as THC[101]
- HU-211, a synthetic cannabinoid derived drug that acts on NMDA instead of endocannabinoid system
- HU-331 a potential anti-cancer drug derived from cannabidiol that specifically inhibits topoisomerase II.
- SR144528, a CB2 receptor antagonist/ inverse agonist[102]
- WIN 55,212-2, a potent cannabinoid receptor agonist
- JWH-133, a potent selective CB2 receptor agonist
- Levonantradol (Nantrodolum), an anti-emetic and analgesic but not currently in use in medicine
- AM-2201, a potent cannabinoid receptor agonist
Recently, the term "neocannabinoid" has been introduced to distinguish these designer drugs from synthetic phytocannabinoids (obtained by chemical synthesis) or synthetic endocannabinoids.[103]
See also
- Cancer and nausea § Cannabinoid
- Cannabinoid receptor antagonist
- Endocannabinoid enhancer
- Endocannabinoid reuptake inhibitor
References
- ↑ "A Proteomic View of Cellular and Molecular Effects of Cannabis". Biomolecules 11 (10): 1411–1428. September 2021. doi:10.3390/biom11101411. PMID 34680044.
- ↑ "Marijuana, also called: Cannabis, Ganja, Grass, Hash, Pot, Weed". 3 July 2017. https://medlineplus.gov/marijuana.html.
- ↑ 3.0 3.1 "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". Journal of Medicinal Chemistry 48 (16): 5059–5087. August 2005. doi:10.1021/jm058183t. PMID 16078824.
- ↑ Cannabinoids. Springer-Verlag. 2005. p. 2. ISBN 978-3-540-22565-2. https://archive.org/details/cannabinoidshand00pert.
- ↑ "Bulletin on Narcotics – 1962 Issue 3 – 004". UNODC (United Nations Office of Drugs and Crime). 1962-01-01. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1962-01-01_3_page005.html.
- ↑ 6.0 6.1 "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products 79 (2): 324–331. February 2016. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472. https://figshare.com/articles/journal_contribution/5028338. Retrieved 2022-12-02.
- ↑ "Phytocannabinoids: Origins and Biosynthesis". Trends in Plant Science 25 (10): 985–1004. October 2020. doi:10.1016/j.tplants.2020.05.005. PMID 32646718.
- ↑ Pate, DW (1999). Anandamide structure-activity relationships and mechanisms of action on intraocular pressure in the normotensive rabbit model. Kuopio University Publications A. Pharmaceutical Sciences Dissertation 37, ISBN:951-781-575-1
- ↑ 9.0 9.1 "Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms". Canadian Family Physician 64 (2): e78–e94. February 2018. PMID 29449262.
- ↑ 10.0 10.1 "Determination and characterization of a cannabinoid receptor in rat brain". Molecular Pharmacology 34 (5): 605–613. November 1988. PMID 2848184. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=2848184. Retrieved 2015-12-24.
- ↑ 11.0 11.1 11.2 "The endocannabinoid system as an emerging target of pharmacotherapy". Pharmacological Reviews 58 (3): 389–462. September 2006. doi:10.1124/pr.58.3.2. PMID 16968947.
- ↑ "Evidence for novel cannabinoid receptors". Pharmacology & Therapeutics 106 (2): 133–145. May 2005. doi:10.1016/j.pharmthera.2004.11.005. PMID 15866316.
- ↑ Medical Physiology: A Cellular and Molecular Approach. Saunders. 2009. p. 331. ISBN 978-1-4160-3115-4.
- ↑ "Effects of cannabis and cannabinoids in the human nervous system.". The effects of drug abuse on the human nervous system. Academic Press. January 2014. pp. 387–422. doi:10.1016/B978-0-12-418679-8.00013-7. ISBN 978-0-12-418679-8.
- ↑ "Localization of cannabinoid CB1 receptors in the human anterior eye and retina". Investigative Ophthalmology & Visual Science 40 (10): 2442–2448. September 1999. PMID 10476817.
- ↑ "Quantitative method to determine mRNA levels by reverse transcriptase-polymerase chain reaction from leukocyte subsets purified by fluorescence-activated cell sorting: application to peripheral cannabinoid receptors". Cytometry 35 (3): 227–234. March 1999. doi:10.1002/(SICI)1097-0320(19990301)35:3<227::AID-CYTO5>3.0.CO;2-4. PMID 10082303.
- ↑ "Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations". European Journal of Biochemistry 232 (1): 54–61. August 1995. doi:10.1111/j.1432-1033.1995.tb20780.x. PMID 7556170.
- ↑ 18.0 18.1 "Is lipid signaling through cannabinoid 2 receptors part of a protective system?". Progress in Lipid Research 50 (2): 193–211. April 2011. doi:10.1016/j.plipres.2011.01.001. PMID 21295074.
- ↑ 19.0 19.1 "Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes". ACS Pharmacology & Translational Science 2 (6): 414–428. December 2019. doi:10.1021/acsptsci.9b00049. PMID 32259074.
- ↑ "Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study". Synapse 53 (4): 208–213. September 2004. doi:10.1002/syn.20050. PMID 15266552.
- ↑ "Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses". European Journal of Biochemistry 268 (6): 1596–1604. March 2001. doi:10.1046/j.1432-1327.2001.02030.x. PMID 11248677.
- ↑ , Andrew D."Controlled cannabis decarboxylization" US patent 20120046352
- ↑ (in en) Cannabis (Marijuana) Research Report (Report). National Institute on Drug Abuse. July 2020. https://nida.nih.gov/publications/research-reports/marijuana/how-does-marijuana-produce-its-effects. Retrieved 2023-05-28.
- ↑ 24.0 24.1 "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophrenia Research 162 (1–3): 153–161. March 2015. doi:10.1016/j.schres.2015.01.033. PMID 25667194.
- ↑ "Cannabidiol--recent advances". Chemistry & Biodiversity 4 (8): 1678–1692. August 2007. doi:10.1002/cbdv.200790147. PMID 17712814.
- ↑ "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology 152 (7): 1092–1101. December 2007. doi:10.1038/sj.bjp.0707460. PMID 17876302.
- ↑ "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research 30 (8): 1037–1043. August 2005. doi:10.1007/s11064-005-6978-1. PMID 16258853.
- ↑ "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367 (1607): 3364–3378. December 2012. doi:10.1098/rstb.2011.0389. PMID 23108553.
- ↑ "Key ingredient staves off marijuana memory loss". Nature. 2010. doi:10.1038/news.2010.508.
- ↑ "Therapeutic Potential of Cannabinoids in Psychosis". Biological Psychiatry 79 (7): 604–612. April 2016. doi:10.1016/j.biopsych.2015.11.018. PMID 26852073.
- ↑ "Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase". Journal of Medicinal Chemistry 40 (20): 3228–3233. September 1997. doi:10.1021/jm970126f. PMID 9379442.
- ↑ Sampson, Peter B. (2021-01-22). "Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the "Big Two"". Journal of Natural Products 84 (1): 142–160. doi:10.1021/acs.jnatprod.0c00965. ISSN 1520-6025. PMID 33356248. https://pubmed.ncbi.nlm.nih.gov/33356248. Retrieved 2022-12-07.
- ↑ "Cannabinol (Code C84510)". NCI Thesaurus. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=ncit&code=C84510.
- ↑ "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology 147 (Suppl 1): S163–S171. January 2006. doi:10.1038/sj.bjp.0706406. PMID 16402100. "Cannabinol (CBN; Figure 1), much of which is thought to be formed from THC during the storage of harvested cannabis, was the first of the plant cannabinoids (phytocannabinoids) to be isolated, from a red oil extract of cannabis, at the end of the 19th century. Its structure was elucidated in the early 1930s by R.S. Cahn, and its chemical synthesis first achieved in 1940 in the laboratories of R. Adams in the U.S.A. and Lord Todd in the U.K.".
- ↑ 35.0 35.1 Pertwee, Roger G (2006). "Cannabinoid pharmacology: the first 66 years: Cannabinoid pharmacology" (in en). British Journal of Pharmacology 147 (S1): S163–S171. doi:10.1038/sj.bjp.0706406. PMID 16402100.
- ↑ 36.0 36.1 Corroon, Jamie (2021-08-31). "Cannabinol and Sleep: Separating Fact from Fiction" (in en). Cannabis and Cannabinoid Research 6 (5): 366–371. doi:10.1089/can.2021.0006. ISSN 2578-5125. PMID 34468204.
- ↑ 37.0 37.1 "CB receptor ligands from plants". Current Topics in Medicinal Chemistry 8 (3): 173–186. 2008. doi:10.2174/156802608783498023. PMID 18289087.
- ↑ "TLC and HPLC Analysis of Alkamides in Echinacea Drugs1,2". Planta Medica 55 (4): 367–371. August 1989. doi:10.1055/s-2006-962030. PMID 17262436.
- ↑ "Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects". The Journal of Biological Chemistry 281 (20): 14192–14206. May 2006. doi:10.1074/jbc.M601074200. PMID 16547349.
- ↑ "Alkamide levels in Echinacea purpurea: a rapid analytical method revealing differences among roots, rhizomes, stems, leaves and flowers". Planta Medica 63 (1): 58–62. February 1997. doi:10.1055/s-2006-957605. PMID 17252329.
- ↑ "Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography–electrospray mass spectrometry". Journal of Chromatography A 815 (2): 205–11. 1998. doi:10.1016/S0021-9673(98)00447-6.
- ↑ "Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB₁ receptor ligand". Pharmacological Research 66 (2): 163–169. August 2012. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- ↑ "Tea catechins' affinity for human cannabinoid receptors". Phytomedicine 17 (1): 19–22. January 2010. doi:10.1016/j.phymed.2009.10.001. PMID 19897346.
- ↑ "Beta-caryophyllene is a dietary cannabinoid". Proceedings of the National Academy of Sciences of the United States of America 105 (26): 9099–9104. July 2008. doi:10.1073/pnas.0803601105. PMID 18574142. Bibcode: 2008PNAS..105.9099G.
- ↑ "Truffles contain endocannabinoid metabolic enzymes and anandamide". Phytochemistry 110: 104–110. February 2015. doi:10.1016/j.phytochem.2014.11.012. PMID 25433633. Bibcode: 2015PChem.110..104P.
- ↑ "Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high". Science Advances 4 (10): eaat2166. October 2018. doi:10.1126/sciadv.aat2166. PMID 30397641. Bibcode: 2018SciA....4.2166C.
- ↑ Muhammad I, Li XC, Jacob MR, Tekwani BL, Dunbar DC, Ferreira D. Antimicrobial and antiparasitic (+)-trans-hexahydrodibenzopyrans and analogues from Machaerium multiflorum. J Nat Prod. 2003 Jun;66(6):804-9. doi:10.1021/np030045o PMID 12828466
- ↑ 48.0 48.1 "Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review". Drug Metabolism Reviews 46 (1): 86–95. February 2014. doi:10.3109/03602532.2013.849268. PMID 24160757. https://zenodo.org/record/1093138. Retrieved 2017-12-07.
- ↑ "Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis". Drug Testing and Analysis 9 (4): 626–633. April 2017. doi:10.1002/dta.1998. PMID 27341312. https://figshare.com/articles/journal_contribution/5028359. Retrieved 2022-12-02.
- ↑ "Pharmacology and effects of cannabis: a brief review". The British Journal of Psychiatry 178 (2): 101–106. February 2001. doi:10.1192/bjp.178.2.101. PMID 11157422. "Because they are extremely lipid soluble, cannabinoids accumulate in fatty tissues, reaching peak concentrations in 4-5 days. They are then slowly released back into other body compartments, including the brain. They are then slowly released back into other body compartments, including the brain. Because of the sequestration in fat, the tissue elimination half-life of THC is about 7 days, and complete elimination of a single dose may take up to 30 days.".
- ↑ "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects". British Journal of Pharmacology 163 (7): 1344–1364. August 2011. doi:10.1111/j.1476-5381.2011.01238.x. PMID 21749363.
- ↑ Malheiro, Rui Filipe; Carmo, Helena; Carvalho, Félix; Silva, João Pedro (January 2023). "Cannabinoid-mediated targeting of mitochondria on the modulation of mitochondrial function and dynamics" (in en). Pharmacological Research 187: 106603. doi:10.1016/j.phrs.2022.106603. PMID 36516885.
- ↑ "Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex®): A Review in Multiple Sclerosis-Related Spasticity". Drugs 77 (5): 563–574. April 2017. doi:10.1007/s40265-017-0720-6. PMID 28293911.
- ↑ 54.0 54.1 "Cannabinoids in the management of difficult to treat pain". Therapeutics and Clinical Risk Management 4 (1): 245–259. February 2008. doi:10.2147/TCRM.S1928. PMID 18728714.
- ↑ "GW Pharmaceuticals launches world's first prescription cannabis drug in Britain". 21 June 2010. https://www.telegraph.co.uk/finance/newsbysector/pharmaceuticalsandchemicals/7842794/GW-Pharmaceuticals-launches-worlds-first-prescription-cannabis-drug-in-Britain.html.
- ↑ "3 prescription drugs that come from marijuana". https://www.usatoday.com/story/money/personalfinance/2014/03/17/three-drugs-that-come-from-marijuana/6531291/.
- ↑ (in de) Neue Arzneimittel. 2011–2012.
- ↑ 58.0 58.1 "FDA and Cannabis: Research and Drug Approval Process". US Food and Drug Administration. 24 February 2023. https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process.
- ↑ "FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy". US Food and Drug Administration. 25 June 2018. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm.
- ↑ "FDA approves first cannabis-based drug". 25 June 2018. https://www.cnn.com/2018/06/25/health/fda-approves-first-cannabis-drug-bn/index.html.
- ↑ "Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine". Cannabinoids 7 (1): 1–11. 2013. http://www.cannabis-med.org/data/pdf/en_2013_01_1.pdf. Retrieved 2017-12-07.
- ↑ "Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L.". The Journal of Supercritical Fluids 129: 16–27. November 2017. doi:10.1016/j.supflu.2017.03.014.
- ↑ "Microextraction techniques for analysis of cannabinoids". TrAC Trends in Analytical Chemistry 80: 156–166. 2016. doi:10.1016/j.trac.2016.03.012.
- ↑ "U.S. Chemist Roger Adams Isolated CBD 75 Years Ago". Fall 2018. https://issuu.com/freedomleaf/docs/freedomleafissue34issuu.
- ↑ "The History Of CBD – A Brief Overview". CBDOrigin.com. 2019-03-08. https://cbdorigin.com/history-of-cbd/.
- ↑ 66.0 66.1 "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology 147 (Suppl 1): S163–S171. January 2006. doi:10.1038/sj.bjp.0706406. PMID 16402100.
- ↑ "Raphael Mechoulam Ph.D.". The Hebrew University of Jerusalem. https://cannabinoids.huji.ac.il/people/raphael-mechoulam.
- ↑ "How I found 'Trips Ahoy' and 'Blackberry Diesel' 'weed' vapes in a state where marijuana is very much illegal". Stat. 23 February 2023. https://www.statnews.com/2023/02/23/easy-to-buy-thc-0-hhc-even-where-marijuana-illegal/.
- ↑ "Delta 8 THC: Everything You Need To Know" (in en-US). 2020-07-09. https://www.laweekly.com/delta-8-thc-everything-you-need-to-know/.
- ↑ "The problems with Cannabinoid Analogs (Delta-8 THC, Delta-10 THC and CBD) and their metabolites detectability in urine drug testing for potential cannabinoid abuse." (in en). USDOJ. 9 December 2021. https://nij.ojp.gov/funding/awards/15pnij-21-gg-04188-ress.
- ↑ Nagarkatti, Prakash; Nagarkatti, Mitzi (28 April 2023). "Cannabis-derived products like delta-8 THC and delta-10 THC have flooded the US market" (in en). USC. https://sc.edu/uofsc/posts/2023/04/conversation_cannabis_derived_products.php.
- ↑ "Delta-8 THC Generated $2 Billion In Revenue In Two Years, Report Finds". Forbes. https://www.forbes.com/sites/dariosabaghi/2023/01/16/delta-8-thc-generated-2-billion-in-revenue-in-2-years-report-finds/?sh=6f49eca34a62.
- ↑ "WHO Expert Committee on Drug Dependence Critical Review". p. 22. https://cdn.who.int/media/docs/default-source/controlled-substances/isomersthc.pdf?sfvrsn=8d45f582_2&download=true.
- ↑ "Delta-8, Delta-10, HHC, THC-O, THCP, and THCV: What should we call these products?". Journal of Studies on Alcohol and Drugs 84 (3): 357–360. March 2023. doi:10.15288/jsad.23-00008. PMID 36971760.
- ↑ "Multiple functions of endocannabinoid signaling in the brain". Annual Review of Neuroscience 35: 529–558. 2012. doi:10.1146/annurev-neuro-062111-150420. PMID 22524785.
- ↑ 76.0 76.1 76.2 "Cannabinoids". Current Drug Targets. CNS and Neurological Disorders 4 (5): 507–530. October 2005. doi:10.2174/156800705774322111. PMID 16266285.
- ↑ "Discovery and characterization of endogenous cannabinoids". Life Sciences 65 (6–7): 573–595. 1999. doi:10.1016/S0024-3205(99)00281-7. PMID 10462059.
- ↑ "Brain cannabinoids in chocolate". Nature 382 (6593): 677–678. August 1996. doi:10.1038/382677a0. PMID 8751435. Bibcode: 1996Natur.382..677D. https://escholarship.org/uc/item/2kk1604c. Retrieved 2022-10-02.
- ↑ "N-Acylethanolamines in seeds. Quantification Of molecular species and their degradation upon imbibition". Plant Physiology 120 (4): 1157–1164. August 1999. doi:10.1104/pp.120.4.1157. PMID 10444099.
- ↑ "Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs. Possible implications for mollusc physiology and sea food industry". Biochimica et Biophysica Acta 1389 (2): 101–111. January 1998. doi:10.1016/S0005-2760(97)00132-X. PMID 9461251.
- ↑ "A second endogenous cannabinoid that modulates long-term potentiation". Nature 388 (6644): 773–778. August 1997. doi:10.1038/42015. PMID 9285589. Bibcode: 1997Natur.388..773S.
- ↑ "Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes". British Journal of Pharmacology 134 (3): 664–672. October 2001. doi:10.1038/sj.bjp.0704297. PMID 11588122.
- ↑ "2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor". Proceedings of the National Academy of Sciences of the United States of America 98 (7): 3662–3665. March 2001. doi:10.1073/pnas.061029898. PMID 11259648. Bibcode: 2001PNAS...98.3662H.
- ↑ "Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species". Journal of Neurochemistry 85 (6): 1374–1381. June 2003. doi:10.1046/j.1471-4159.2003.01804.x. PMID 12787057.
- ↑ "N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo". The Biochemical Journal 351 Pt 3 (3): 817–824. November 2000. doi:10.1042/bj3510817. PMID 11042139.
- ↑ "The endocannabinoid signalling system: biochemical aspects". Pharmacology, Biochemistry, and Behavior 81 (2): 224–238. June 2005. doi:10.1016/j.pbb.2005.01.027. PMID 15935454.
- ↑ "Cannabinoid modulation of peripheral autonomic and sensory neurotransmission". European Journal of Pharmacology 472 (1–2): 1–21. July 2003. doi:10.1016/S0014-2999(03)01813-2. PMID 12860468.
- ↑ "Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor". The Journal of Pharmacology and Experimental Therapeutics 301 (3): 1020–1024. June 2002. doi:10.1124/jpet.301.3.1020. PMID 12023533. http://pdfs.semanticscholar.org/ab53/846ea9f65d5a673d2e4552933c2a26409b00.pdf.
- ↑ "Lysophosphatidylinositol signalling: new wine from an old bottle". Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1821 (4): 694–705. April 2012. doi:10.1016/j.bbalip.2012.01.009. PMID 22285325. https://zenodo.org/record/895487. Retrieved 2019-09-13.
- ↑ "What to know about endocannabinoids and the endocannabinoid system". 27 February 2021. https://www.medicalnewstoday.com/articles/endocannabinoid.
- ↑ "Retrograde signaling at central synapses via endogenous cannabinoids". Molecular Psychiatry 7 (3): 234–235. 2002. doi:10.1038/sj.mp.4000999. PMID 11920149.
- ↑ "Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood". Experimental Biology and Medicine 230 (4): 225–234. April 2005. doi:10.1177/153537020523000401. PMID 15792943.
- ↑ "Effects of cannabinoids (marijuana) on taste intensity and hedonic ratings and salivary flow of adults". Chemical Senses 19 (2): 125–140. April 1994. doi:10.1093/chemse/19.2.125. PMID 8055263.
- ↑ "Endocannabinoids selectively enhance sweet taste". Proceedings of the National Academy of Sciences of the United States of America 107 (2): 935–939. January 2010. doi:10.1073/pnas.0912048107. PMID 20080779. Bibcode: 2010PNAS..107..935Y.
- ↑ "Retrograde signalling by endocannabinoids". Cannabinoids. Handbook of Experimental Pharmacology. 168. 2005. pp. 367–383. doi:10.1007/3-540-26573-2_12. ISBN 3-540-22565-X.
- ↑ "Getting to the Bottom of the Runner's High" (in en-US). The New York Times. 2021-03-10. ISSN 0362-4331. https://www.nytimes.com/2021/03/10/well/move/running-exercise-mental-effects.html.
- ↑ "Synthesis of the individual, pharmacologically distinct, enantiomers of a tetrahydrocannabinol derivative". Tetrahedron: Asymmetry 1 (5): 315–318. 1990. doi:10.1016/S0957-4166(00)86322-3.
- ↑ "Synthetic cannabinoids: analysis and metabolites". Life Sciences. Special Issue: Emerging Trends in the Abuse of Designer Drugs and Their Catastrophic Health Effects: Update on Chemistry, Pharmacology, Toxicology and Addiction Potential 97 (1): 78–90. February 2014. doi:10.1016/j.lfs.2013.12.212. PMID 24412391.
- ↑ "Comparison of outcome expectancies for synthetic cannabinoids and botanical marijuana". The American Journal of Drug and Alcohol Abuse 42 (4): 377–384. July 2016. doi:10.3109/00952990.2015.1135158. PMID 26910181.
- ↑ 100.0 100.1 "N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide(AB-CHMINACA), N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA)and[1-(5-fluoropentyl)-1H-indazol-3-yl(naphthalen-1-yl)methanone(THJ-2201)"]. Drug and Chemical Evaluation Section, Office of Diversion Control, Drug Enforcement Administration. December 2014. http://www.grassley.senate.gov/sites/default/files/news/upload/3-factor%20analysis%20AB-CHMINACA%20AB-PINACA%20THJ2201%2012172014.pdf.
- ↑ "More medicinal uses for marijuana". Marijuana.org. October 18, 2005. http://www.marijuana.org/mydna10-12-05.htm.
- ↑ "SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor". The Journal of Pharmacology and Experimental Therapeutics 284 (2): 644–650. February 1998. PMID 9454810.
- ↑ "'Cannabis' ontologies I: Conceptual issues with Cannabis and cannabinoids terminology" (in en). Drug Science, Policy and Law 6: 25–29. 2020. doi:10.1177/2050324520945797. ISSN 2050-3245.
External links
 |