Chemistry:Demegestone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lutionex |
| Other names | Dimegestone; R-2453; RU-2453; 17α-Methyl-δ9-19-norprogesterone; 17α-Methyl-19-norpregna-4,9-diene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Good[2] |
| Metabolism | Hydroxylation, others[2] |
| Metabolites | • 21-Hydroxydemegestone[2] • Others[2] |
| Excretion | Urine[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed.[3][4][5][6][7] It is taken by mouth.[2][1]
Demegestone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[6][2][8] It has no androgenic activity.[2]
Demegestone was first described in 1966 and was introduced for medical use in France in 1974.[3][4] It has only been marketed in France, and has since been discontinued in this country.[5][4]
Medical uses
Demegestone has been used to treat luteal insufficiency.[7] It has also been studied in combination with estrogens, such as moxestrol, as an oral contraceptive and treatment for infertility.[1][9][10]
Side effects
Pharmacology
Pharmacodynamics
Demegestone is a progestogen, and hence is an agonist of the progesterone receptor (PR).[6][8][2] It is a highly potent progestogen, showing 50 times the potency of progesterone in the Clauberg test.[2] The ovulation-inbhiting dosage of demegestone is 2.5 mg/day, while the endometrial transformation dosage is 100 mg per cycle.[11] The medication is devoid of androgenic activity,[2] and instead has some antiandrogenic activity.[12] Demegestone has low affinity for the glucocorticoid receptor.[13] In a particular bioassay, both demegestone and progesterone showed antiglucocorticoid rather than glucocorticoid activity.[14] The major metabolite of demegestone, a 21-hydroxylated metabolite, is a moderately potent progestogen (4 times the potency of progesterone) and a weak mineralocorticoid (2% of the potency of deoxycorticosterone).[2]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Demegestone | 230 | 1 | 0 | 5 | 1–2 | ? | ? |
Pharmacokinetics
Demegestone has good bioavailability.[2] The initial volume of distribution of demegestone is 31 L.[2] Demegestone is metabolized by hydroxylation at the C21, C1, C2, and C11 positions, which is eventually followed by A-ring aromatization after 1,2-dehydration.[2] The major metabolite of demegestone is a 21-hydroxy derivative.[2] The metabolic clearance rate of demegestone is 20 L/h.[2] Its biological half-lives are 2.39 and 0.24 hours with intravenous injection.[2] Demegestone and/or its metabolites are excreted, at least in part, in urine.[2]
Chemistry
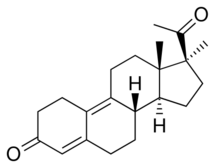
Demegestone, also known as 17α-methyl-δ9-19-norprogesterone or as 17α-methyl-19-norpregna-4,9-diene-3,20-dione, is a synthetic norpregnane steroid and a derivative of progesterone.[3][4][6] It is specifically a combined derivative of 17α-methylprogesterone and 19-norprogesterone, or of 17α-methyl-19-norprogesterone.[3][4][6] Related derivatives of 17α-methyl-19-norprogesterone include promegestone and trimegestone.[3][6]
History
Demegestone was first described in the literature in 1964 and was introduced for medical use in 1974 in France .[3][4] It was developed by Roussel Uclaf.[4]
Society and culture
Generic names
Demegestone is the generic name of the drug and its INN.[3] It is also known by its developmental code name R-2453 or RU-2453.[3]
Brand names
Demegestone was marketed under the brand name Lutionex.[3][4]
Availability
Demegestone is no longer marketed and hence is no longer available in any country.[5] It was previously available in France .[5][4]
References
- ↑ 1.0 1.1 1.2 "Evaluation of a low-dose progestagen as a contraceptive". Nihon Funin Gakkai Zasshi 16 (1): 68–82. 1971. PMID 12158578.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 "121. Metabolic studies of R2453, a highly potent progestin". Journal of Steroid Biochemistry 5 (4): 324. 1974. doi:10.1016/0022-4731(74)90266-0. ISSN 0022-4731.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 356–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA356.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 1215–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1215.
- ↑ 5.0 5.1 5.2 5.3 "Demegestone". Micromedex. http://www.micromedexsolutions.com/micromedex2/.: [yes|permanent dead link|dead link}}]
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ 7.0 7.1 "[Luteal insufficiency and elevation of sex-binding proteins by demegestone]" (in fr). Revue Francaise de Gynecologie et d'Obstetrique 83 (7–9): 495–498. 1988. PMID 3194612.
- ↑ 8.0 8.1 "Quantitative relationships between steroid structure and binding to putative progesterone receptors". Journal of Medicinal Chemistry 20 (9): 1139–1146. September 1977. doi:10.1021/jm00219a006. PMID 926114.
- ↑ "[Clinical observation on oral contraceptive effect by R-2453 (Abstracts of Papers Presented at Showa 44 in the field of gynecology)."]. Japanese Journal of Obstetrics and Gynecology-Acta Obstetrica et Gynaecologica Japonica 22 (7): 753. 1970. https://ci.nii.ac.jp/naid/110002126113/.
- ↑ "Treatment of Ovarian Sterility with Combined Moxestrol-Demegestone Preparation.". Journal de Gynecologie Obstetrique et Biologie de la Reproduction (Paris, France: Masson Editeur) 8 (1): 89. January 1979.
- ↑ "Oral Contraceptive Pills: Combinations, Dosages and the Rationale behind 50 Years or Oral Hormonal Contraceptive Development.". Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology 8 (1): 58–129. October 2011. http://www.kup.at/kup/pdf/10166.pdf.
- ↑ "Steroid hormones—agonists and antagonists". Mechanisms of Steroid Action. 1981. pp. 145–158. doi:10.1007/978-1-349-81345-2_11. ISBN 978-1-349-81347-6.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid7382482 - ↑ "The relationship between glucocorticoid structure and effects upon thymocytes". Molecular Pharmacology 13 (5): 948–955. September 1977. PMID 895725.
 |

