Chemistry:Norpropoxyphene
| |
| Names | |
|---|---|
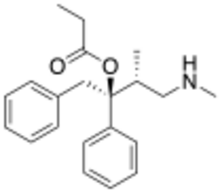
| Preferred IUPAC name
(2S,3R)-3-Methyl-4-(methylamino)-1,2-diphenylbutan-2-yl propanoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H27NO2 | |
| Molar mass | 325.445 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Norpropoxyphene is a major metabolite of the opioid analgesic drug dextropropoxyphene,[1] and is responsible for many of the side effects associated with use of this drug, especially the unusual toxicity seen during dextropropoxyphene overdose.[2][3][4] It has weaker analgesic effects than dextropropoxyphene itself, but is a relatively potent pro-convulsant and blocker of sodium and potassium channels,[5] particularly in heart tissue,[6][7] which produces prolonged intracardiac conduction time and can lead to heart failure following even relatively minor overdoses.[8][9][10][11] The toxicity of this metabolite makes dextropropoxyphene up to 10 times more likely to cause death following overdose compared to other similar mild opioid analgesics,[12] and has led to dextropropoxyphene being withdrawn from the market in some countries.[13]
Because norpropoxyphene has a long half-life in the body of up to 36 hours (compared to around 6–12 hours for dextropropoxyphene), it can accumulate in tissues during chronic use of dextropropoxyphene-containing medications, especially in people whose excretion of drugs is slower than normal such as young children, the elderly, and individuals with reduced kidney or liver function, and so side effects including serious adverse events are more common in these groups and use of dextropropoxyphene should be avoided where possible.[14][15][16]
References
- ↑ "CYP3A4 mediates dextropropoxyphene N-demethylation to nordextropropoxyphene: human in vitro and in vivo studies and lack of CYP2D6 involvement". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 34 (10): 875–87. October 2004. doi:10.1080/00498250400008371. PMID 15764408.
- ↑ "Acute poisoning with dextropropoxyphene. Clinical symptoms and plasma concentrations". Acta Medica Scandinavica 200 (4): 241–8. 1976. doi:10.1111/j.0954-6820.1976.tb08226.x. PMID 983792.
- ↑ "Propoxyphene and norpropoxyphene concentrations in blood and tissues in cases of fatal overdose". Clinical Chemistry 22 (8): 1319–21. August 1976. doi:10.1093/clinchem/22.8.1319. PMID 949842.
- ↑ "Pharmacologic and toxic effects in animals of dextropropoxyphene and its major metabolite norpropoxyphene: a review". Human Toxicology 3 Suppl: 13S–36S. August 1984. doi:10.1177/096032718400300103. PMID 6090306.
- ↑ "Propoxyphene and norpropoxyphene: pharmacologic and toxic effects in animals". The Journal of Pharmacology and Experimental Therapeutics 200 (1): 245–53. January 1977. PMID 13200.
- ↑ "Cardio-respiratory toxicity of propoxyphene and norpropoxyphene in conscious rabbits". Acta Pharmacologica et Toxicologica 42 (3): 171–8. March 1978. doi:10.1111/j.1600-0773.1978.tb02187.x. PMID 580345.
- ↑ "Norpropoxyphene-induced cardiotoxicity is associated with changes in ion-selectivity and gating of HERG currents". Cardiovascular Research 44 (3): 568–78. December 1999. doi:10.1016/s0008-6363(99)00258-8. PMID 10690289.
- ↑ "Dextropropoxyphene overdosage. Pharmacological considerations and clinical management". Drugs 26 (1): 70–9. July 1983. doi:10.2165/00003495-198326010-00004. PMID 6349964.
- ↑ "Dextropropoxyphene overdose. Epidemiology, clinical presentation and management". Medical Toxicology and Adverse Drug Experience 2 (6): 430–44. 1987. doi:10.1007/BF03259877. PMID 3323775.
- ↑ "ECG abnormalities in co-proxamol (paracetamol/dextropropoxyphene) poisoning". Clinical Toxicology 43 (4): 255–9. 2005. doi:10.1081/CLT-66069. PMID 16035201.
- ↑ "Co-proxamol and suicide: preventing the continuing toll of overdose deaths". QJM 98 (3): 159–70. March 2005. doi:10.1093/qjmed/hci026. PMID 15728397.
- ↑ "Co-proxamol overdose is associated with a 10-fold excess mortality compared with other paracetamol combination analgesics". British Journal of Clinical Pharmacology 60 (4): 444–7. October 2005. doi:10.1111/j.1365-2125.2005.02468.x. PMID 16187978.
- ↑ "Co-proxamol withdrawal has reduced suicide from drugs in Scotland". British Journal of Clinical Pharmacology 66 (2): 290–3. August 2008. doi:10.1111/j.1365-2125.2008.03206.x. PMID 18489609.
- ↑ "Propoxyphene and norpropoxyphene plasma concentrations in the anephric patient". Clinical Pharmacology and Therapeutics 27 (5): 665–70. May 1980. doi:10.1038/clpt.1980.94. PMID 7371364.
- ↑ "Propoxyphene and norpropoxyphene kinetics after single and repeated doses of propoxyphene". Clinical Pharmacology and Therapeutics 31 (2): 157–67. February 1982. doi:10.1038/clpt.1982.25. PMID 7056023.
- ↑ "Pharmacokinetics of dextropropoxyphene and nordextropropoxyphene in young and elderly volunteers after single and multiple dextropropoxyphene dosage". British Journal of Clinical Pharmacology 28 (4): 463–9. October 1989. doi:10.1111/j.1365-2125.1989.tb03527.x. PMID 2590604.
 |

