Chemistry:Estriol 3-glucuronide
From HandWiki
Short description: Chemical compound
| |
| Names | |
|---|---|
| IUPAC name
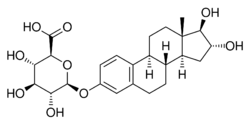
16α,17β-Dihydroxyestra-1,3,5(10)-trien-3-yl β-D-glucopyranosiduronic acid
| |
| Systematic IUPAC name
(2S,3S,4S,5R,6S)-6-{[(1R,2R,3aS,3bR,9bS,11aS)-1,2-Dihydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-7-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| Other names
(16α,17β)-16,17-Dihydroxyestra-1(10),2,4-trien-3-yl β-D-glucopyranosiduronic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C24H32O9 | |
| Molar mass | 464.511 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Estriol 3-glucuronide, or oestriol 3-glucuronide, also known as estriol 3-β-D-glucosiduronic acid, is a natural, steroidal estrogen and a glucuronic acid (β-D-glucopyranuronic acid) conjugate of estriol.[1] It is found in the urine of women as a reversibly formed metabolite of estriol.[2][3] The positional isomer of estriol 3-glucuronide, estriol 16α-glucuronide, also occurs as an endogenous metabolite of estriol, but to a much greater extent in comparison.[2][3]
See also
- Estrogen conjugate
- Estradiol glucuronide
- Estrone glucuronide
- Estradiol sulfate
- Estrone sulfate
- Lipoidal estradiol
- Catechol estrogen
References
- ↑ R.A. Hill; H.L.J. Makin; D.N. Kirk; G.M. Murphy (23 May 1991). Dictionary of Steroids. CRC Press. pp. 274–. ISBN 978-0-412-27060-4. https://books.google.com/books?id=AI7EnUyeEtUC&pg=PA274.
- ↑ 2.0 2.1 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 265–. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA265.
- ↑ 3.0 3.1 Musey, Paul I.; Kirdani, Rashad Y.; Bhanalaph, Thongchai; Sandberg, Avery A. (1973). "Estriol metabolism in the baboon: Analysis of urinary and biliary metabolites". Steroids 22 (6): 795–817. doi:10.1016/0039-128X(73)90054-8. ISSN 0039-128X. PMID 4203562.
External links
 |

