Chemistry:Osaterone acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Ypozane |
| Other names | TZP-4238; Gestoxarone acetate; 2-Oxachloromadinone acetate; 17α-Acetoxy-6-chloro-2-oxa-6-dehydroprogesterone; 17α-Acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione |
| Routes of administration | By mouth (tablets) |
| Drug class | Steroidal antiandrogen; Progestogen; Progestin; Progestogen ester |
| Pharmacokinetic data | |
| Protein binding | Osaterone acetate: 90% 15β-Hydroxyosaterone acetate: 80%[1] (Both mainly to albumin)[1] |
| Metabolism | Liver[1] |
| Metabolites | 15β-Hydroxyosaterone acetate[1] |
| Elimination half-life | Dogs: 80 hours to 197 ± 109 hours[1][2] |
| Excretion | Bile: 60%[1] Urine: 25%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
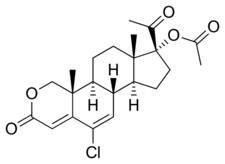
| Formula | C22H27ClO5 |
| Molar mass | 406.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs.[1][3][4] It is given by mouth.[1]
Osaterone acetate is an antiandrogen, and hence is an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone.[1] It is also a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1]
Osaterone acetate was introduced for veterinary use in 2007.[5] It is marketed in Europe.[6][1]
Uses
Veterinary
Osaterone acetate is used in veterinary medicine in Europe in the treatment of benign prostatic hyperplasia (BPH) in dogs.[1][3][4] It has been found to produce remission of clinical symptoms of BPH in 83% of dogs for six months after a single one-week course of treatment,[7] and can be used long-term.[4]
Available forms
Osaterone acetate comes in the form of 1.875 mg, 3.75 mg, 7.5 mg, and 15 mg oral tablets for veterinary use.[1]
Side effects
Side effects of osaterone acetate include diminished sperm quality (for up to 6 weeks post-treatment), transient elevation of liver enzymes (caution should be observed with known liver disease), vomiting, diarrhea, polyuria/polydipsia, lethargy, and hyperplasia of the mammary glands.[8] It can also decrease cortisol levels, interfere with adrenocorticotropic hormone response, induce or exacerbate adrenal insufficiency, and exacerbate diabetes mellitus.[9][8]
Pharmacology
Pharmacodynamics
Osaterone acetate is a steroidal antiandrogen, progestin, and antigonadotropin.[1] It has virtually no estrogenic or androgenic activity.[3] Its side-effect profile indicates that it possesses clinically relevant glucocorticoid activity.[9][8] An active metabolite of osaterone acetate, 15β-hydroxyosaterone acetate, has potent antiandrogenic activity similarly to osaterone acetate.[1] Osaterone acetate treats BPH in dogs by reducing the actions of androgens in the prostate gland.[1]
Pharmacokinetics
The major active metabolite of osaterone acetate is 15β-hydroxyosaterone acetate.[1] Osaterone acetate has a long biological half-life of 80 hours to 197 ± 109 hours in dogs.[1][2]
Chemistry
Osaterone acetate, also known as 2-oxachloromadinone acetate, as well as 17α-acetoxy-6-chloro-2-oxa-6-dehydroprogesterone or 17α-acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[6] It is a derivative of the less potent chlormadinone acetate.[3] The medication is the C17α acetate ester of osaterone.[6]
History
Osaterone acetate was introduced for veterinary use in Europe under the brand name Ypozane in 2007.[6][5][1]
Society and culture
Generic names
Osaterone acetate is the generic name of the drug.[6] Osaterone is the INN of the deacetylated parent compound.[6]
Brand names
Osaterone acetate is marketed under the brand name Ypozane by Virbac.[6]
Availability
Osaterone acetate is available widely throughout Europe, including in Belgium, Finland , France , Germany , Italy, the Netherlands, Norway , Poland , Sweden, Switzerland , and the United Kingdom .[6]
Research
Osaterone acetate was also investigated in Japan in the treatment of prostate cancer and BPH in humans but was ultimately never marketed for such purposes.[3][10]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 "Ypozane for Dogs". European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/veterinary/000112/WC500069537.pdf.
- ↑ 2.0 2.1 Small Animal Clinical Pharmacology. Elsevier Health Sciences. 2008. pp. 536–. ISBN 978-0-7020-2858-8. https://books.google.com/books?id=RpsROVqemk8C&pg=PA536.
- ↑ 3.0 3.1 3.2 3.3 3.4 Molecular Therapies of Cancer. Springer. 22 July 2015. pp. 316–. ISBN 978-3-319-13278-5. https://books.google.com/books?id=dhs_CgAAQBAJ&pg=PA316.
- ↑ 4.0 4.1 4.2 Canine Reproduction and Neonatology. Teton NewMedia. 18 December 2014. pp. 296–. ISBN 978-1-4987-2850-8. https://books.google.com/books?id=GlFsBgAAQBAJ&pg=PA296.
- ↑ 5.0 5.1 "Neue Arzneimittel für Kleintiere 2007". Tierärztliche Praxis Ausgabe K: Kleintiere/Heimtiere. 36 (5): 311–22. 2008. doi:10.1055/s-0038-1622691.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 "Osaterone". Drugs.com. https://www.drugs.com/international/osaterone.html.
- ↑ Clinical Veterinary Advisor: Dogs and Cats. Elsevier Health Sciences. 9 December 2014. pp. 848–. ISBN 978-0-323-24074-1. https://books.google.com/books?id=NmziBQAAQBAJ&pg=PA848.
- ↑ 8.0 8.1 8.2 Theriogenology, An Issue of Veterinary Clinics: Small Animal Practice. Elsevier Health Sciences. 28 May 2012. pp. 112–. ISBN 978-1-4557-4447-3. https://books.google.com/books?id=gtoRaSR2NxcC&pg=PT112.
- ↑ 9.0 9.1 Textbook of Veterinary Internal Medicine. Elsevier Health Sciences. 24 December 2009. pp. 2055–. ISBN 978-1-4377-0282-8. https://books.google.com/books?id=4Qzau1jagOYC&pg=PA2055.
- ↑ "Steroidal Antiandrogens". Hormone Therapy in Breast and Prostate Cancer. Cancer Drug Discovery and Development. Humana Press. 2009. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
Further reading
- "Steroidal Antiandrogens". Hormone Therapy in Breast and Prostate Cancer. Cancer Drug Discovery and Development. Humana Press. 2009. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
External links
 |

