Chemistry:Etiocholanolone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Aetiocholanolone 5-Isoandrosterone |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
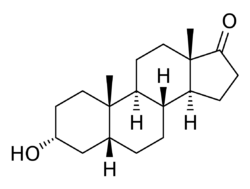
| Formula | C19H30O2 |
| Molar mass | 290.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etiocholanolone, also known as 5β-androsterone, as well as 3α-hydroxy-5β-androstan-17-one or etiocholan-3α-ol-17-one, is an etiocholane (5β-androstane) steroid as well as an endogenous 17-ketosteroid that is produced from the metabolism of testosterone. It causes fever, immunostimulation, and leukocytosis, and is used to evaluate adrenal cortex function, bone marrow performance, and in neoplastic disease to stimulate the immune system. Etiocholanolone is also known to be an inhibitory androstane neurosteroid,[1] acting as a positive allosteric modulator of the GABAA receptor,[2] and possesses anticonvulsant effects.[3] The unnatural enantiomer of etiocholanolone is more potent as a positive allosteric modulator of GABAA receptors and as an anticonvulsant than the natural form.[4]
Etiocholanolone has been studied as a pyrogenic steroid in the so-called steroid fever (or etiocholanolone fever),[5][6] a condiditon similar to familial mediterranean fever (FMF). Etiocholanolone (like pregnanolone) activates the pyrin inflammasome.[7] It is not known whether these endogenous steroids play a role in triggering FMF flares but they may make a link between stress, menstrual cycle and disease flares.[8][9]
Etiocholanolone is produced from 5β-dihydrotestosterone, with 3α,5β-androstanediol as an intermediate.
Chemistry
See also
References
- ↑ Reddy DS (2010). "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. 186. 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 9780444536303.
- ↑ "Natural and enantiomeric etiocholanolone interact with distinct sites on the rat alpha1beta2gamma2L GABAA receptor". Molecular Pharmacology 71 (6): 1582–1590. June 2007. doi:10.1124/mol.106.033407. PMID 17341652.
- ↑ "Anticonvulsant activity of androsterone and etiocholanolone". Epilepsia 46 (6): 819–827. June 2005. doi:10.1111/j.1528-1167.2005.00705.x. PMID 15946323.
- ↑ "Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms". Psychopharmacology 231 (17): 3325–3332. September 2014. doi:10.1007/s00213-014-3546-x. PMID 24705905.
- ↑ "Steroid fever". The Lancet 281 (7285): 835–836. April 1963. doi:10.1016/S0140-6736(63)91549-6.
- ↑ "Novel biological properties of steroid metabolites; fever-production in man". Journal of the Reticuloendothelial Society 4 (4): 231–236. July 1967. PMID 6056839.
- ↑ "Steroid hormone catabolites activate the pyrin inflammasome through a non-canonical mechanism". Cell Reports 41 (2): 111472. October 2022. doi:10.1016/j.celrep.2022.111472. PMID 36223753.
- ↑ "The factors considered as trigger for the attacks in patients with familial Mediterranean fever". Rheumatology International 33 (4): 893–897. April 2013. doi:10.1007/s00296-012-2453-x. PMID 22814791.
- ↑ "The relations between attacks and menstrual periods and pregnancies of familial Mediterranean fever patients". Rheumatology International 26 (7): 676–679. May 2006. doi:10.1007/s00296-005-0041-z. PMID 16184383.
 |

