Biology:Coagulation factor II receptor
 Generic protein structure example |
Proteinase-activated receptor 1 (PAR1) also known as protease-activated receptor 1 or coagulation factor II (thrombin) receptor is a protein that in humans is encoded by the F2R gene.[1] PAR1 is a G protein-coupled receptor and one of four protease-activated receptors involved in the regulation of thrombotic response. Highly expressed in platelets and endothelial cells, PAR1 plays a key role in mediating the interplay between coagulation and inflammation, which is important in the pathogenesis of inflammatory and fibrotic lung diseases.[2] It is also involved both in disruption and maintenance of endothelial barrier integrity, through interaction with either thrombin or activated protein C, respectively.[3]
Structure
PAR1 is a transmembrane G-protein-coupled receptor (GPCR) that shares much of its structure with the other protease-activated receptors.[4][5] These characteristics include having seven transmembrane alpha helices, four extracellular loops and three intracellular loops.[5] PAR1 specifically contains 425 amino acid residues arranged for optimal binding of thrombin at its extracellular N-terminus. The C-terminus of PAR1 is located on the intracellular side of the cell membrane as part of its cytoplasmic tail.[4]
Signal transduction pathway
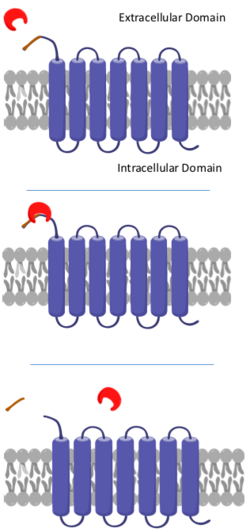
Activation
PAR1 is activated when the terminal 41 amino acids of its N-terminus are cleaved by thrombin, a serine protease.[6] Thrombin recognizes PAR1 by a Lysine-Aspartate-Proline-Arginine-Serine sequence at the N-terminal, where it cuts the peptide bond between Arginine-41 and Serine-42. The affinity of thrombin to this specific cleavage site in PAR1 is further aided by secondary interactions between thrombin's exosite and an acidic region of amino acid residues located C-terminal to Ser-42.[7] This proteolytic cleavage is irreversible and the loose peptide, often referred to as parstatin, is then released outside of the cell.[6] The newly revealed N-terminus acts as a tethered ligand that binds to a binding region between extracellular loops 3 and 4 of PAR1, therefore activating the protein. The binding instigates conformational changes in the protein that ultimately allow for the binding of G-proteins to sites on the intracellular region of PAR1.[8]
Signalling
Once cleaved, PAR1 can activate G-proteins that bind to several locations on its intracellular loops. For example, PAR1 in conjunction with PAR4 can couple to and activate G-protein G12/13 which in turn activates Rho and Rho kinase.[4] This pathway leads to the quick alteration of platelet shape due to actin contractions that lead to platelet mobility, as well as the release of granules which are both necessary for platelet aggregation.[4] Coupling can also occur with Gq, leading to phospholipase C-β activation; this pathway results in the stimulation of protein kinase C (PKC) which impacts platelet activation.[4]
Additionally, both PAR1 and PAR4 can couple to G-protein q which stimulates intracellular movement for Calcium ions that serve as second messengers for platelet activation.[4] This also activates protein kinase C which stimulates platelet aggregation and therefore blood coagulation further down the pathway.[7]
Termination
The phosphorylation of PAR1's cytoplasmic tail and subsequent binding to arrestin uncouples the protein from G protein signaling.[6][7] These phosphorylated PAR1s are transported back into the cell via endosomes where they are sent to Golgi bodies. The cleaved PAR1s are then sorted and transported to lysosomes where they are degraded.[7] This internalization and degradation process is necessary for the termination of receptor signaling.[6]
In order to regain thrombin responsiveness, PAR1 must be replenished in the cell surface. Uncleaved PAR1 in the cell membrane gets bound by the AP2 adaptor complex at a tyrosine motif on the intracellular C-terminus, which stimulates the endocytosis of the unactivated PAR1.[9] It is then stored in clathrin-coated vesicles within the cytosol and ultimately protected from proteolysis. This ensures that there is a constant supply of uncleaved PAR1 that can be cycled into the plasma membrane independent of PAR1 reproduction, thus resensitizing the cell to thrombin and resetting the signal transduction pathway.[10]
Ligands
Agonists
Finding selective agonists for PAR1 has also been a topic of interest for researchers. A synthetic SFLLRN peptide has been found to serve as an agonist for PAR1. The SFLLRN peptide mimics the first six residues of the N-terminal tethered ligand of activated PAR1 and binds to the same binding site on the second extracellular loop.[11] So, even in the absence of thrombin, SFLLRN binding can garner a response from cleaved or uncleaved PAR1.[12]
Antagonists
Selective antagonists for the PAR1 receptor have been developed for use as anti-clotting agents.
- SCH-79797
- Vorapaxar, sold under the brand name Zontivity, is a first-in-class anti-platelet drug used in the treatment of heart disease in patients with a history of heart attacks and peripheral artery disease.[13] Vorapaxar has been recently shown to attenuate the neutrophilic inflammatory response to Streptococcus pneumoniae by reducing levels of pro-inflammatory cytokines such as IL-1β and chemokines CXCL1, CCL2 and CCL7.[14] PAR1 is inhibited by Vorapaxar when the molecule binds to a binding pocket between extracellular loop 2 and 3 of the PAR1 where it stabilizes the inactivated protein structure and prevents the switch to the active conformation.[11]
See also
References
- ↑ "Chromosomal assignment of the human thrombin receptor gene: localization to region q13 of chromosome 5". Blood 82 (5): 1532–7. September 1993. doi:10.1182/blood.V82.5.1532.1532. PMID 8395910.
- ↑ ""Proteinase-activated receptors in fibroproliferative lung disease". Thorax 69 (2): 190–2. February 2014. doi:10.1136/thoraxjnl-2013-204367. PMID 24186921.
- ↑ "Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation". Blood 105 (8): 3178–84. April 2005. doi:10.1182/blood-2004-10-3985. PMID 15626732.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Michelson, Alan D. (2013). Platelets (3rd ed.). Amsterdam: Elsevier. ISBN 9780123878380. OCLC 820818942.
- ↑ 5.0 5.1 "Structural Properties of the Human Protease-Activated Receptor 1 Changing by a Strong Antagonist". Structure 26 (6): 829–838.e4. June 2018. doi:10.1016/j.str.2018.03.020. PMID 29731231.
- ↑ 6.0 6.1 6.2 6.3 "Signal transduction by protease-activated receptors". British Journal of Pharmacology 160 (2): 191–203. May 2010. doi:10.1111/j.1476-5381.2010.00705.x. PMID 20423334.
- ↑ 7.0 7.1 7.2 7.3 "Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer". Journal of Cell Science 120 (Pt 6): 921–8. March 2007. doi:10.1242/jcs.03409. PMID 17344429.
- ↑ "Identifying and quantifying two ligand-binding sites while imaging native human membrane receptors by AFM". Nature Communications 6 (1): 8857. November 2015. doi:10.1038/ncomms9857. PMID 26561004. Bibcode: 2015NatCo...6.8857P.
- ↑ "Regulation of protease-activated receptor 1 signaling by the adaptor protein complex 2 and R4 subfamily of regulator of G protein signaling proteins". The Journal of Biological Chemistry 289 (3): 1580–91. January 2014. doi:10.1074/jbc.m113.528273. PMID 24297163.
- ↑ "Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization". Molecular and Cellular Biology 26 (8): 3231–42. April 2006. doi:10.1128/MCB.26.8.3231-3242.2006. PMID 16581796.
- ↑ 11.0 11.1 "High-resolution crystal structure of human protease-activated receptor 1". Nature 492 (7429): 387–92. December 2012. doi:10.1038/nature11701. PMID 23222541. Bibcode: 2012Natur.492..387Z.
- ↑ "Protease-activated receptor-1 can mediate responses to SFLLRN in thrombin-desensitized cells: evidence for a novel mechanism for preventing or terminating signaling by PAR1's tethered ligand". Biochemistry 38 (8): 2486–93. February 1999. doi:10.1021/bi982527i. PMID 10029543.
- ↑ "Vorapaxar: The Current Role and Future Directions of a Novel Protease-Activated Receptor Antagonist for Risk Reduction in Atherosclerotic Disease". Drugs in R&D 17 (1): 65–72. March 2017. doi:10.1007/s40268-016-0158-4. PMID 28063023.
- ↑ "Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection". Journal of Immunology 194 (12): 6024–34. June 2015. doi:10.4049/jimmunol.1500124. PMID 25948816.
Further reading
- "Characterization of a functional thrombin receptor. Issues and opportunities". The Journal of Clinical Investigation 89 (2): 351–5. February 1992. doi:10.1172/JCI115592. PMID 1310691.
- "Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: correlation with brain edema". Neurochemistry International 57 (3): 248–53. October 2010. doi:10.1016/j.neuint.2010.06.002. PMID 20541575.
- "Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis". Biochemical Society Transactions 30 (2): 211–6. April 2002. doi:10.1042/BST0300211. PMID 12023853.
- "Role and regulation of the thrombin receptor (PAR-1) in human melanoma". Oncogene 22 (20): 3130–7. May 2003. doi:10.1038/sj.onc.1206453. PMID 12789289.
- "PGE2 and PAR-1 in pulmonary fibrosis: a case of biting the hand that feeds you?". American Journal of Physiology. Lung Cellular and Molecular Physiology 288 (5): L789-92. May 2005. doi:10.1152/ajplung.00016.2005. PMID 15821019.
- "Protease-activated receptors in cardiovascular diseases". Circulation 114 (10): 1070–7. September 2006. doi:10.1161/CIRCULATIONAHA.105.574830. PMID 16952995.
- "Protease-activated receptor signaling: new roles and regulatory mechanisms". Current Opinion in Hematology 14 (3): 230–5. May 2007. doi:10.1097/MOH.0b013e3280dce568. PMID 17414212.
- "Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation". Cell 64 (6): 1057–68. March 1991. doi:10.1016/0092-8674(91)90261-V. PMID 1672265.
- "Solid tumor cells express functional "tethered ligand" thrombin receptor". Cancer Research 55 (3): 698–704. February 1995. PMID 7834643.
- "Intracellular targeting and trafficking of thrombin receptors. A novel mechanism for resensitization of a G protein-coupled receptor". The Journal of Biological Chemistry 269 (44): 27719–26. November 1994. doi:10.1016/S0021-9258(18)47045-7. PMID 7961693.
- "Crystallographic structures of thrombin complexed with thrombin receptor peptides: existence of expected and novel binding modes". Biochemistry 33 (11): 3266–79. March 1994. doi:10.1021/bi00177a018. PMID 8136362.
- "G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets". Proceedings of the National Academy of Sciences of the United States of America 91 (2): 504–8. January 1994. doi:10.1073/pnas.91.2.504. PMID 8290554. Bibcode: 1994PNAS...91..504O.
- "Response of blood leukocytes to thrombin receptor peptides". Journal of Leukocyte Biology 54 (2): 145–51. August 1993. doi:10.1002/jlb.54.2.145. PMID 8395550.
- "Genomic cloning and characterization of the human thrombin receptor gene. Structural similarity to the proteinase activated receptor-2 gene". The Journal of Biological Chemistry 271 (16): 9307–12. April 1996. doi:10.1074/jbc.271.16.9809. PMID 8621593.
- "Cloning and identification of regulatory sequences of the human thrombin receptor gene". The Journal of Biological Chemistry 271 (42): 26320–8. October 1996. doi:10.1074/jbc.271.42.26320. PMID 8824285.
- "Role of the thrombin receptor's cytoplasmic tail in intracellular trafficking. Distinct determinants for agonist-triggered versus tonic internalization and intracellular localization". The Journal of Biological Chemistry 271 (51): 32874–80. December 1996. doi:10.1074/jbc.271.51.32874. PMID 8955127.
- "Direct evidence for two distinct G proteins coupling with thrombin receptors in human neuroblastoma SH-EP cells". European Journal of Pharmacology 316 (1): 105–9. November 1996. doi:10.1016/S0014-2999(96)00653-X. PMID 8982657.
- "Thrombin receptors on human platelets. Initial localization and subsequent redistribution during platelet activation". The Journal of Biological Chemistry 272 (9): 6011–7. February 1997. doi:10.1074/jbc.272.9.6011. PMID 9038223.
- "Specific inhibition of thrombin-induced cell activation by the neutrophil proteinases elastase, cathepsin G, and proteinase 3: evidence for distinct cleavage sites within the aminoterminal domain of the thrombin receptor". Blood 89 (6): 1944–53. March 1997. doi:10.1182/blood.V89.6.1944. PMID 9058715.
External links
- Overview of all the structural information available in the PDB for UniProt: P25116 (Proteinase-activated receptor 1) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |

