Biology:Retinal G protein coupled receptor
 Generic protein structure example |
RPE-retinal G protein-coupled receptor also known as RGR-opsin is a protein that in humans is encoded by the RGR gene.[1][2] RGR-opsin is a member of the rhodopsin-like receptor subfamily of GPCR. Like other opsins which bind retinaldehyde, it contains a conserved lysine residue in the seventh transmembrane domain.[3][4] RGR-opsin comes in different isoforms produced by alternative splicing.[2]
Function
RGR-opsin preferentially binds all-trans-retinal,[4] which is the dominant form in the dark adapted retina, upon light exposure it is isomerized to 11-cis-retinal.[5] Therefore, RGR-opsin presumably acts as a photoisomerase to convert all-trans-retinal to 11-cis-retinal, similar to retinochrome in invertebrates. 11-cis-retinal is isomerized back within rhodopsin and the iodopsins in the rods and cones of the retina. RGR-opsin is exclusively expressed in tissue close to the rods and cones, the retinal pigment epithelium (RPE) and Müller cells.[3]
Phylogeny
The RGR-opsins are restricted to the echinoderms, the hemichordates the craniates.[6] The craniates are the taxon that contains mammals and with them humans. The RGR-opsins are one of the seven subgroups of the chromopsins. The other groups are the peropsins, the retinochromes, the nemopsins, the astropsins, the varropsins, and the gluopsins.[6] The chromopsins are one of three subgroups of the tetraopsins (also known as RGR/Go or Group 4 opsins). The other groups are the neuropsins and the Go-opsins. The tetraopsins are one of the five major groups of the animal opsins, also known as type 2 opsins). The other groups are the ciliary opsins (c-opsins, cilopsins), the rhabdomeric opsins (r-opsins, rhabopsins), the xenopsins, and the nessopsins. Four of these subclades occur in Bilateria (all but the nessopsins).[6][7] However, the bilaterian clades constitute a paraphyletic taxon without the opsins from the cnidarians.[6][7][8][9]
-
Phylogenetic reconstruction of the opsins. The outgroup contains other G protein-coupled receptors. The frame highlights the tetraopsins, which are expanded in the next image.
-
Phylogenetic reconstruction of the tetraopsins. The outgroup contains other G protein-coupled receptors including the other opsins. The frame highlights the chromopsins, which are expanded in the next image.
-
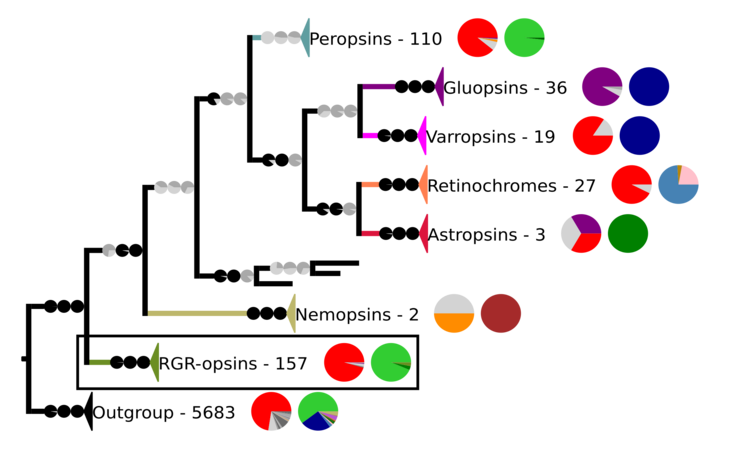
Phylogenetic reconstruction of the chromopsins. The outgroup contains other G protein-coupled receptors including the other opsins. The frame highlights the RGR-opsins.
In the phylogeny above, Each clade contains sequences from opsins and other G protein-coupled receptors. The number of sequences and two pie charts are shown next to the clade. The first pie chart shows the percentage of a certain amino acid at the position in the sequences corresponding to position 296 in cattle rhodopsin. The amino acids are color-coded. The colors are red for lysine (K), purple for glutamic acid (E), orange for arginine (R), dark and mid-gray for other amino acids, and light gray for sequences that have no data at that position. The second pie chart gives the taxon composition for each clade, green stands for craniates, dark green for cephalochordates, mid green for echinoderms, brown for nematodes, pale pink for annelids, dark blue for arthropods, light blue for mollusks, and purple for cnidarians. The branches to the clades have pie charts, which give support values for the branches. The values are from right to left SH-aLRT/aBayes/UFBoot. The branches are considered supported when SH-aLRT ≥ 80%, aBayes ≥ 0.95, and UFBoot ≥ 95%. If a support value is above its threshold the pie chart is black otherwise gray.[6]
Clinical significance
RGR-opsin may be associated with autosomal recessive and autosomal dominant retinitis pigmentosa (arRP and adRP, respectively).[10][2]
Interactions
RGR-opsin has been shown to interact with KIAA1279.[11]
References
- ↑ "Localization of the human RGR opsin gene to chromosome 10q23". Human Genetics 97 (6): 720–2. Jun 1996. doi:10.1007/BF02346179. PMID 8641686.
- ↑ 2.0 2.1 2.2 "Entrez Gene: RGR retinal G protein coupled receptor". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5995.
- ↑ 3.0 3.1 "An opsin homologue in the retina and pigment epithelium". Investigative Ophthalmology & Visual Science 34 (13): 3669–78. Dec 1993. PMID 8258527.
- ↑ 4.0 4.1 "A human opsin-related gene that encodes a retinaldehyde-binding protein". Biochemistry 33 (44): 13117–25. Nov 1994. doi:10.1021/bi00248a022. PMID 7947717.
- ↑ Hao, W; Fong, HK (5 March 1999). "The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium.". The Journal of Biological Chemistry 274 (10): 6085–90. doi:10.1074/jbc.274.10.6085. PMID 10037690.
- ↑ 6.0 6.1 6.2 6.3 6.4 "The Gluopsins: Opsins without the Retinal Binding Lysine". Cells 11 (15): 2441. August 2022. doi:10.3390/cells11152441. PMID 35954284.
 Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 7.0 7.1 "The last common ancestor of most bilaterian animals possessed at least 9 opsins". Genome Biology and Evolution: evw248. 26 October 2016. doi:10.1093/gbe/evw248. PMID 27797948.
- ↑ "Shedding new light on opsin evolution". Proceedings. Biological Sciences 279 (1726): 3–14. January 2012. doi:10.1098/rspb.2011.1819. PMID 22012981.
- ↑ "Cubozoan genome illuminates functional diversification of opsins and photoreceptor evolution". Scientific Reports 5: 11885. July 2015. doi:10.1038/srep11885. PMID 26154478. Bibcode: 2015NatSR...511885L.
- ↑ "Mutations in RGR, encoding a light-sensitive opsin homologue, in patients with retinitis pigmentosa". Nature Genetics 23 (4): 393–4. Dec 1999. doi:10.1038/70496. PMID 10581022.
- ↑ "Towards a proteome-scale map of the human protein-protein interaction network". Nature 437 (7062): 1173–8. Oct 2005. doi:10.1038/nature04209. PMID 16189514. Bibcode: 2005Natur.437.1173R.
Further reading
- "Alternative splicing in human retinal mRNA transcripts of an opsin-related protein". Experimental Eye Research 60 (4): 401–6. Apr 1995. doi:10.1016/S0014-4835(05)80096-X. PMID 7789419.
- "A human opsin-related gene that encodes a retinaldehyde-binding protein". Biochemistry 33 (44): 13117–25. Nov 1994. doi:10.1021/bi00248a022. PMID 7947717.
- "Interaction of 11-cis-retinol dehydrogenase with the chromophore of retinal g protein-coupled receptor opsin". The Journal of Biological Chemistry 276 (24): 21098–104. Jun 2001. doi:10.1074/jbc.M010441200. PMID 11274198.
- "Synthesis of the all-trans-retinal chromophore of retinal G protein-coupled receptor opsin in cultured pigment epithelial cells". The Journal of Biological Chemistry 277 (5): 3318–24. Feb 2002. doi:10.1074/jbc.M108946200. PMID 11723126.
- "In silico characterisation and chromosomal localisation of human RRH (peropsin)--implications for opsin evolution". BMC Genomics 4 (1): 3. Jan 2003. doi:10.1186/1471-2164-4-3. PMID 12542842.
- "Expression of opsin genes early in ocular development of humans and mice". Experimental Eye Research 76 (3): 393–6. Mar 2003. doi:10.1016/S0014-4835(02)00300-7. PMID 12573668.
- "Study of the involvement of the RGR, CRPB1, and CRB1 genes in the pathogenesis of autosomal recessive retinitis pigmentosa". Journal of Medical Genetics 40 (7): 89e–89. Jul 2003. doi:10.1136/jmg.40.7.e89. PMID 12843338.
- "Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene". The Journal of Biological Chemistry 281 (31): 22289–98. Aug 2006. doi:10.1074/jbc.M602664200. PMID 16737970.
- "Deposition of exon-skipping splice isoform of human retinal G protein-coupled receptor from retinal pigment epithelium into Bruch's membrane". Molecular Vision 13: 1203–14. 2007. PMID 17679941.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |