Chemistry:Esmodafinil
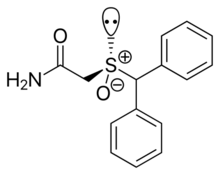 (S)-(+)-modafinil
| |
| Names | |
|---|---|
| IUPAC name
2-[(S)-benzhydrylsulfinyl]acetamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H15NO2S | |
| Molar mass | 273.35 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+317Script error: No such module "Preview warning".Category:GHS errors, P330, P501 | |
| Related compounds | |
Related compounds
|
Modafinil, Armodafinil |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Esmodafinil (also known as (S)-modafinil or (+)-Modafinil) is the enantiopure isolation of the (S) enantiomer of modafinil. Unlike armodafinil, esmodafinil has never been marketed on its own.[2]
Esmodafinil is suspected to be less clinically useful for treating conditions that modafinil and armodafinil are marketed for, such as narcolepsy, sleep-shift work disorder, and obstructive sleep apnea.[3]
Pharmacology
Pharmacokinetics
Esmodafinil possesses a substantially shorter half life (3–5 hours) compared to armodafinil (10–13 hours).[4]
Pharmacodynamics
Esmodafinil has a 3 times lower affinity for the dopamine transporter compared to armodafinil.[4] Both enantiomers of modafinil preferentially bind to the dopamine transporter in an inward facing conformation.[4][5]
Preclinical research
Esmodafinil has been researched for the treatment of cocaine addiction.[4][5] Like armodafinil, esmodafinil attenuates the effects of cocaine by occupying the dopamine transporter.[5] While doing so, esmodafinil increases dopamine in the nucleus accumbens to a lesser extent than cocaine.[4] However, the short half-life of esmodafinil has been cited as reason to investigate armodafinil as a cocaine addiction treatment instead.[4]
Analysis in biological samples
Modafinil is considered a stimulant doping agent and as such is prohibited by World Anti-Doping Agency in sports competitions.[6] The validated methods are researched to separately quantify modafinil enantiomers in the real samples.[7]
References
- ↑ "(S)-Modafinil" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11173366#section=Safety-and-Hazards.
- ↑ "NCATS Inxight Drugs — MODAFINIL, (S)-" (in en). https://drugs.ncats.io/drug/152JRG3T0U.
- ↑ Tembe, D. V.; Dhavale, A.; Desai, H.; Mane, D. N.; Raut, S. K.; Dhingra, G.; Sardesai, U.; Saoji, S. et al. (2011-06-01). "Armodafinil versus Modafinil in Patients of Excessive Sleepiness Associated with Shift Work Sleep Disorder: A Randomized Double Blind Multicentric Clinical Trial" (in en). Neurology Research International 2011: e514351. doi:10.1155/2011/514351. ISSN 2090-1852. PMID 21766023.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Loland, Claus J.; Mereu, Maddalena; Okunola, Oluyomi M.; Cao, Jianjing; Prisinzano, Thomas E.; Mazier, Sonia; Kopajtic, Theresa; Shi, Lei et al. (September 2012). "R-Modafinil (Armodafinil): A Unique Dopamine Uptake Inhibitor and Potential Medication for Psychostimulant Abuse" (in en). Biological Psychiatry 72 (5): 405–413. doi:10.1016/j.biopsych.2012.03.022. PMID 22537794.
- ↑ 5.0 5.1 5.2 Schmitt, Kyle C.; Reith, Maarten E. A. (2011). "The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors". PLOS ONE 6 (10): e25790. doi:10.1371/journal.pone.0025790. ISSN 1932-6203. PMID 22043293. Bibcode: 2011PLoSO...625790S.
- ↑ "The Prohibited List". https://www.wada-ama.org/en/prohibited-list.
- ↑ "New enantioselective LC method development and validation for the assay of modafinil". J Pharm Biomed Anal 138: 267–271. May 2017. doi:10.1016/j.jpba.2017.02.028. PMID 28231529.
 |

