Chemistry:Para-Chloromethamphetamine
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
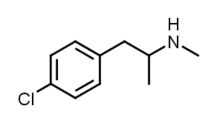
| Formula | C10H14ClN |
| Molar mass | 183.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
para-Chloromethamphetamine (also known as 4-chloromethamphetamine and 4-CMA) is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA).[1][2] It has been found to decrease serotonin in rats.[3][4][5] Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.[6]
Another study on rats found that 4-chloromethamphetamine was more potent at inducing conditioned taste aversion than methamphetamine.[7]
4-Chloromethamphetamine was further investigated in the 1960s along with 4-CA and it was noted that they differed from their parent amphetamine and methamphetamine substances by exhibiting only a slight central stimulant effect in both animals and humans and that they acted like antidepressants rather than stimulants.[8][9][10][11]

Studies in the 1970s found that a single dose of 10 mg/1 kg 4-CMA resulted in a decreased level of 5-hydroxytryptamine in the brain for several weeks.[12]
4-Chloromethamphetamine was identified outside of the laboratory for the first time at the Tomorrowland festival edition 2015, where a tablet was found in possession of a drug dealer (see picture).[13] In the following year, tablets with 4–CMA were also found in Romania, Austria and Croatia. Fortituously, and for unknown reasons, 4-CMA disappeared briefly from European rave scene after the Spring of 2016. However, a 2019 study of participants of dance music festival in Belgium reported detection of 4-CMA in pills (out of 178 analyzed samples only one was mostly 4-CMA, while in one other 4-CMA was a minor ingredient).[14]
See also
- 3-Chloromethamphetamine
- 4-Bromoamphetamine
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-Iodoamphetamine
References
- ↑ "[3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues". European Journal of Pharmacology 200 (1): 9–16. July 1991. doi:10.1016/0014-2999(91)90659-E. PMID 1685125.
- ↑ "Comparison of 4-chloro-, 4-bromo- and 4-fluoroamphetamine in rats: drug levels in brain and effects on brain serotonin metabolism". Neuropharmacology 14 (10): 739–746. October 1975. doi:10.1016/0028-3908(75)90099-4. PMID 1196472.
- ↑ "Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry". Psychopharmacology 220 (3): 495–508. April 2012. doi:10.1007/s00213-011-2504-0. PMID 21993877.
- ↑ "Decrease of cerebral 5-hydroxytryptamine and 5-hydroxyindolacetic acid by an arylalkylamine". Life Sciences 2 (11): 828–833. November 1963. doi:10.1016/0024-3205(63)90094-8. PMID 14078137.
- ↑ "Lowering of brain serotonin level by chloramphetamines". Biochemical Pharmacology 14 (4): 483–488. April 1965. doi:10.1016/0006-2952(65)90221-2. PMID 14322972.
- ↑ "Long-term effects of p-chloroamphetamine and related drugs on central serotonergic mechanisms". The Journal of Pharmacology and Experimental Therapeutics 192 (1): 33–41. January 1975. PMID 1123726. http://jpet.aspetjournals.org/content/192/1/33.short.
- ↑ "Comparative potencies of amphetamine, fenfluramine and related compounds in taste aversion experiments in rats". British Journal of Pharmacology 61 (4): 669–677. December 1977. doi:10.1111/j.1476-5381.1977.tb07560.x. PMID 597669.
- ↑ "Influencing the human indoleamine metabolism by means of a chlorinated amphetamine derivative with antidepressive action (p-chloro-N-methylamphetamine)". Psychopharmacologia 13 (2): 145–160. July 1968. doi:10.1007/BF00404812. PMID 5678577.
- ↑ "A controlled study of the antidepressant effect of p-Chloro-N-methylamphetamine, a compound with a selective effect on the central 5-hydroxytryptamine metabolism". Acta Psychiatrica Scandinavica 46 (4): 365–373. 1970. doi:10.1111/j.1600-0447.1970.tb02126.x. PMID 5502782.
- ↑ "Investigation into the possible influence of chlorinated amphetamine derivatives on 5-hydroxytryptamine synthesis in man". Psychopharmacologia 18 (4): 412–420. December 1970. doi:10.1007/BF00402767. PMID 4923523.
- ↑ "A comparative study of the therapeutic effects of sone 4-chlorinated amphetamine derivatives in depressive patients". Psychopharmacologia 20 (1): 66–76. March 1971. doi:10.1007/BF00404060. PMID 5565748.
- ↑ Long-term effects of p-chloroamphetamine and related drugs on central serotonergic mechanisms. 1975. Journal of Pharmacology and Experimental Therapeutics. 192/1, 33-41. E. Sanders-Bush, J.A. Bushing, F. Sulser.
- ↑ "Identification and characterization of 4-chloromethamphetamine (4-CMA) in seized ecstacy - a risk to public health". Forensic Science International 288: 173–180. July 2018. doi:10.1016/j.forsciint.2018.04.023. PMID 29753935.
- ↑ Lessons to be learned from toxicological analyses in intoxicated patients and seized materials at an electronic music dance festival. 2019. Forensic Sci Int. 299/174-9. P. Calle, K. Maudens, S. Lemoyne, S. Geerts, D. Van Sassenbroeck, P. Jensen, et al. doi: 10.1016/j.forsciint.2019.03.047.
 |
