Chemistry:Lomevactone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
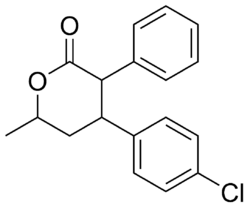
| Formula | C18H17ClO2 |
| Molar mass | 300.78 g·mol−1 |
| 3D model (JSmol) | |
| |
Lomevactone (INN; developmental code name DR-250) is a drug described as a psychostimulant and antidepressant which was synthesized and assayed in the 1980s, but was never marketed.[1][2]
Stereoisomers
There are eight possible stereoisomers of lomevactone. It is the (3R,4R,6R)-form that has the psychotherapeutic properties.[3][4]
Synthesis
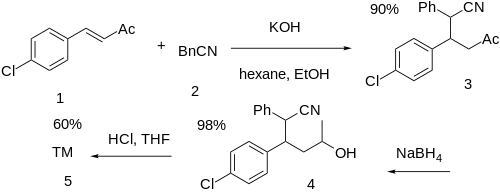
The conjugate 1,4-alkylation reaction between 4-chlorobenzylideneacetone (1) and phenylacetonitrile (2) gives 3-(4-chlorophenyl)-5-oxo-2-phenylhexanenitrile (3). The selective reduction of the keto group to the alcohol with sodium borohydride gives 3-(4-chlorophenyl)-5-hydroxy-2-phenylhexanenitrile (4). Hydrolysis of the nitrile to an acid gives 3-(4-chlorophenyl)-5-hydroxy-2-phenylhexanoic acid. This is followed by lactone formation completing the synthesis of lomevactone (5).
References
- ↑ David J. Triggle (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9. https://books.google.com/books?id=A0THacd46ZsC&q=lomevactone&pg=PA1231.
- ↑ "The progressive ratio schedule as a model for studying the psychomotor stimulant activity of drugs in the rat". Psychopharmacology 80 (2): 184–9. 1983. doi:10.1007/BF00427967. PMID 6136063.
- ↑ Axiotis, S.; Druex, J.; Perrin, M.; Royer, J. (1982). "Conformations in the tetrahydropyran-2-one ring". Tetrahedron. 38 (4): 499–504. doi:10.1016/0040-4020(82)80093-8.
- ↑ "Tétrahydropyrones-2 III. Recherche d'une activité psychostimulante spécifique". European Journal of Medicinal Chemistry 22 (4): 293–303. July 1987. doi:10.1016/0223-5234(87)90266-2.
- ↑ Axiotis, S. et al, Eur. J. Med. Chem.-Chim. Ther., 1981, 16, 431, 439.
- ↑ Pierre Simon & Jacques Dreux, U.S. Patent 4,287,206 (1981 to Sanofi Aventis France).
- ↑ 시몽 삐에르 & 드로 짝끄, KR830002288 (1983).
 |

