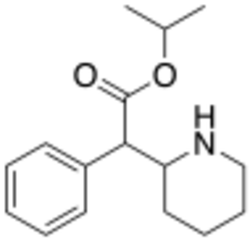
Chemistry:Isopropylphenidate
From HandWiki
Short description: Stimulant designer drugs
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Isopropylphenidate (also known as IPH and IPPD) is a piperidine based stimulant drug, closely related to methylphenidate, but with the methyl ester replaced by an isopropyl ester. It has similar effects to methylphenidate but with a longer duration of action,[1][2] and was banned in the UK as a Temporary Class Drug from April 2015 following its unapproved sale as a designer drug.[3]
It has been researched as potential methylphenidate replacement for ADHD and narcolepsy, because of fewer side effects.[4][2]
See also
References
- ↑ "Isopropylphenidate: an ester homolog of methylphenidate with sustained and selective dopaminergic activity and reduced drug interaction liability". Journal of Child and Adolescent Psychopharmacology 23 (10): 648–54. December 2013. doi:10.1089/cap.2013.0074. PMID 24261661. https://deepblue.lib.umich.edu/bitstream/2027.42/140321/1/cap.2013.0074.pdf.
- ↑ 2.0 2.1 Markowitz JS, Patrick KS, Zhu H, "Isopropylphenidate for Treatment of Attention-Deficit/Hyperactivity Disorder and Fatigue-Related Disorders and Conditions", US patent application 20120245201, published 2012-09-27
- ↑ "Methylphenidate-based NPS: A review of the evidence of use and harm.". Advisory Council on the Misuse of Drugs. 31 March 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/420983/TCDO_methylphenidate_NPS.pdf.
- ↑ Florence Levy (July 2014). "Applications of pharmacogenetics in children with attention-deficit/hyperactivity disorder". Pharmgenomics Pers Med: 349–356.
 |
