Chemistry:S33005
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
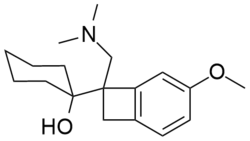
| Other names | (–)-1-(1-dimethylaminomethyl) 5-methoxybenzocyclobutan-1-yl) cyclohexanol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H27NO2 |
| Molar mass | 289.419 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
S33005 is a serotonin–norepinephrine reuptake inhibitor (SNRI) that was under development by Servier for the treatment of depression and related disorders. It is structurally related to venlafaxine but has a more complex molecular structure. Venlafaxine appears to be a sigma modulator,[1] but it is not known if S33005 shares this activity.
Synthesis
"The 1-cyano-benzocyclobutenes used as starting material are obtained, for example, by subjecting a β-[orthohalogeno-phenyl]-propionitrile to intramolecular condensation in the presence of potassium amide, or by brominating a benzocyclobutene in position 1 with N-bromosuccinimide, followed by exchange of the bromine atom for a cyano group by means of sodium cyanide."[2]
See also
References
- ↑ "Involvement of sigma-1 receptor modulation in the antidepressant action of venlafaxine". Neuroscience Letters 420 (3): 204–8. June 2007. doi:10.1016/j.neulet.2007.04.055. PMID 17532136.
- ↑ U.S. Patent 3,622,614
External links
- PubMed search
- Binding Database
- The patent and synthesis discussion can be found in U.S. Patent 6,107,345
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 | 0.00      (0 votes) (0 votes) |