Chemistry:Chlorobutanol
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1,1-Trichloro-2-methylpropan-2-ol | |||
| Other names
1,1,1-Trichloro-2-methyl-2-propanol; Chlorbutol; Chloreton; Chloretone; Chlortran; Trichloro-tert-butyl alcohol; 1,1,1-Trichloro-tert-butyl alcohol; 2-(Trichloromethyl)propan-2-ol; tert-Trichlorobutyl alcohol; Trichloro-tert-butanol; Trichlorisobutylalcohol; 2,2,2-Trichloro-1,1-dimethylethanol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H7Cl3O | |||
| Molar mass | 177.45 g·mol−1 | ||
| Appearance | White solid | ||
| Odor | Camphor | ||
| Melting point | 95–99 °C (203–210 °F; 368–372 K) | ||
| Boiling point | 167 °C (333 °F; 440 K) | ||
| Slightly soluble | |||
| Solubility in acetone | Soluble | ||
| Pharmacology | |||
| 1=ATC code }} | A04AD04 (WHO) | ||
| Hazards | |||
| Main hazards | Xn | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
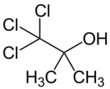
Chlorobutanol (trichloro-2-methyl-2-propanol) is an organic compound with the formula CCl
3C(OH)(CH
3)
2. The compound is an example of a chlorohydrin. The compound is a preservative, sedative, hypnotic and weak local anesthetic similar in nature to chloral hydrate. It has antibacterial and antifungal properties.[1] Chlorobutanol is typically used at a concentration of 0.5% where it lends long term stability to multi-ingredient formulations. However, it retains antimicrobial activity at 0.05% in water. Chlorobutanol has been used in anesthesia and euthanasia of invertebrates and fishes.[2][3] It is a white, volatile solid with a camphor-like odor.
Synthesis
Chlorobutanol was first synthesized in 1881 by the German chemist Conrad Willgerodt (1841–1930).[4]
Chlorobutanol is formed by the reaction of chloroform and acetone in the presence of potassium or sodium hydroxide. It may be purified by sublimation or recrystallisation.[5]
Parthenogenesis
Chlorobutanol has proven effective at stimulating parthenogenesis in sea urchin eggs up to the pluteus stage, possibly by increasing irritability to cause stimulation. For the eggs of the fish Oryzias latipes, however, chlorobutanol only acted as an anesthetic.[6]
Pharmacology and toxicity
It is an anesthetic with effects related to isoflurane and halothane.[7]
Chlorobutanol is toxic to the liver, a skin irritant and a severe eye irritant.[8]
References
- ↑ Noecker, Robert (2001). "Effects of common ophthalmic preservatives on ocular health". Advances in Therapy 18 (5): 205–215. doi:10.1007/bf02853166. PMID 11783457.
- ↑ W. N. McFarland & G. W. Klontz (1969). "Anesthesia in fishes". Federation Proceedings 28 (4): 1535–1540. PMID 4894939.
- ↑ John E. Cooper (2011). "Anesthesia, analgesia, and euthanasia of invertebrates". ILAR Journal 52 (2): 196–204. doi:10.1093/ilar.52.2.196. PMID 21709312.
- ↑ See:
- Willgerodt, C. (1881). "Ueber die Einwirkung ätzender Alkalien auf halogenirte Verbindungen in Acetonlösungen" (in de). Berichte der Deutschen Chemischen Gesellschaft 14 (2): 2451–2460. doi:10.1002/cber.188101402191. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hzb&view=1up&seq=873.
- Willgerodt, C. (1882). "Ueberführung des Acetonchloroforms in die Oxyisobuttersäure" (in de). Berichte der Deutschen Chemischen Gesellschaft 15 (2): 2305–2308. doi:10.1002/cber.188201502176. https://babel.hathitrust.org/cgi/pt?id=uiug.30112025692838&view=1up&seq=1205.
- Willgerodt, C. (1883). "Zur Kenntniss des Acetonchloroforms" (in de). Berichte der Deutschen Chemischen Gesellschaft 16 (2): 1585. doi:10.1002/cber.18830160218. https://babel.hathitrust.org/cgi/pt?id=osu.32435060218708&view=1up&seq=469.
- Willgerodt, C.; Genieser, Ad. (1888). "Beiträge zur Kenntniss des Acetonchloroforms" (in de). Journal für praktische Chemie 37 (8): 361–374. doi:10.1002/prac.18880370131. https://babel.hathitrust.org/cgi/pt?id=mdp.39015026393622&view=1up&seq=377.
- ↑ "Chlorobutanol". http://www.sciencemadness.org/smwiki/index.php/Chlorobutanol#Preparation.
- ↑ Embryologia 1956
- ↑ Nicholas P. Franks (2006). "Molecular targets underlying general anaesthesia". British Journal of Pharmacology 147 (Suppl 1): S72–S81. doi:10.1038/sj.bjp.0706441. PMID 16402123.
- ↑ MSDS
External links
 |