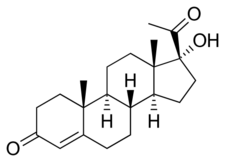
Chemistry:17α-Hydroxyprogesterone
| |
| |
| Names | |
|---|---|
| IUPAC name
17α-Hydroxypregn-4-ene-3,20-dione
| |
| Systematic IUPAC name
(1R,3aS,3bR,9aR,9bS,11aS)-1-Acetyl-1-hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
Hydroxyprogesterone (INN)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.46 g/mol |
| Melting point | 219.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP),[1] or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone.[2][3][4] It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.
Biological activity
17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison.[5] In addition, it is an antagonist of the mineralocorticoid receptor (MR)[6] as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone.[5][7][8]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ? | |||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, dexamethasone for the GR, and estradiol for the ER. Sources: See template. | ||||||||
Biochemistry
Biosynthesis
17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1)[citation needed]
17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production.[citation needed]
This steroid is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[9][10]
Measurement
Measurements of levels of 17α-OHP are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17α-OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17α-OHP. 17α-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but note, 17α-OHP is also contributed by the placenta.[citation needed]
Immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) used to clinically determine 17α-OHP are prone to cross-reactivity with the 17α-OHP steroid precursors and their sulphated conjugates. Gas or liquid chromatography and mass spectrometry (e.g. LC-MS/MS) achieves greater specificity than immunoassays.[11][12]
Measurement of 17α-OHP by LC-MS/MS improves newborn screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency, because 17α-OHP steroid precursors and their sulphated conjugates which are present in the first two days after birth and longer in pre-term neonates, cross-react in immunoassays with 17α-OHP, giving falsely high 17α-OHP levels.[11][12]
Pharmacology
Pharmacokinetics
Although 17α-OHP has not been used as a medication, its pharmacokinetics have been studied and reviewed.[13]
Medical uses
Esters of 17α-OHP, such as hydroxyprogesterone caproate and, to a far lesser extent, hydroxyprogesterone acetate and hydroxyprogesterone heptanoate, have been used in medicine as progestins.[2][3][4] When "hydroxyprogesterone" is referenced from the standpoint of medical use, what is usually being referred to is actually, in general, hydroxyprogesterone caproate.[citation needed]
Chemistry
17α-OHP, also known as 17α-hydroxypregn-4-ene-3,20-dione, is a naturally occurring pregnane steroid. It features ketone groups at the C3 and C20 positions, a hydroxyl group at the C17α position, and a double bond between the C4 and C5 positions.[citation needed]
17α-OHP is the parent compound of a class of progestins referred to as the 17α-hydroxyprogesterone derivatives.[14][15][16] Among others, this class of drugs includes chlormadinone acetate, cyproterone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[14][15][16]
Society and culture
Generic names
Hydroxyprogesterone is the generic name of 17α-OHP and its INN and BAN.[2][3][4]
See also
- 11α-Hydroxyprogesterone
- 5α-Dihydroprogesterone
- 20-Dihydroprogesterone
- 11-Deoxycorticosterone
- 11-Deoxycortisol
- 17α-Methylprogesterone
- 19-Norprogesterone
- 19-Nortestosterone
References
- ↑ "17-hydroxyprogesterone (17OHP)". https://www.gloshospitals.nhs.uk/our-services/services-we-offer/pathology/tests-and-investigations/17-hydroxyprogesterone-17ohp/.
- ↑ 2.0 2.1 2.2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 664–665. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA665.
- ↑ 3.0 3.1 3.2 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 146–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA146.
- ↑ 4.0 4.1 4.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA532.
- ↑ 5.0 5.1 "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. 2007. doi:10.1016/j.ajog.2007.05.024. PMID 18060946.
- ↑ "Influence of 17-Hydroxyprogesterone, Progesterone and Sex Steroids on Mineralocorticoid Receptor Transactivation in Congenital Adrenal Hyperplasia". Horm Res Paediatr 83 (6): 414–421. April 2015. doi:10.1159/000374112. PMID 25896481. http://pure-oai.bham.ac.uk/ws/files/25244128/Mooij_et_al_Influence_17_Hydroxyprogesterone_Hormone_Research_Paediatrics_2015_Post_Print.pdf.
- ↑ "Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia lead to Transactivation of the Glucocorticoid Receptor". Endocrinology 156 (10): 3504–3510. 2015. doi:10.1210/en.2015-1087. PMID 26207344.
- ↑ Sun, Kang; Lei, Kaiyu; Chen, Li; Georgiou, Ektoras X.; Sooranna, Suren R.; Khanjani, Shirin; Brosens, Jan J.; Bennett, Phillip R. et al. (2012). "Progesterone Acts via the Nuclear Glucocorticoid Receptor to Suppress IL-1β-Induced COX-2 Expression in Human Term Myometrial Cells". PLOS ONE 7 (11): e50167. doi:10.1371/journal.pone.0050167. ISSN 1932-6203. PMID 23209664. Bibcode: 2012PLoSO...750167L.
- ↑ Reference Values During Pregnancy
- ↑ "normal ranges for hormone tests in women". http://www.keratin.com/ab/ab012.shtml.
- ↑ 11.0 11.1 "Measurement of 17-Hydroxyprogesterone by LCMSMS Improves Newborn Screening for CAH Due to 21-Hydroxylase Deficiency in New Zealand". International Journal of Neonatal Screening 6 (1): 6. March 2020. doi:10.3390/ijns6010006. PMID 33073005.
- ↑ 12.0 12.1 "Wisconsin's Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia". International Journal of Neonatal Screening 5 (3): 33. September 2019. doi:10.3390/ijns5030033. PMID 33072992.
- ↑ Die Gestagene. Springer-Verlag. 27 November 2013. pp. 276–277. ISBN 978-3-642-99941-3. https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA276.
- ↑ 14.0 14.1 Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7. https://books.google.com/books?id=BWMeSwVwfTkC&pg=PA289.
- ↑ 15.0 15.1 Robert Alan Prentky; Ann Wolbert Burgess (31 July 2000). Forensic Management of Sexual Offenders. Springer Science & Business Media. pp. 219–. ISBN 978-0-306-46278-8. https://books.google.com/books?id=-50Of8_n_TAC&pg=PA219.
- ↑ 16.0 16.1 H. J. Smith; Hywel Williams (1 January 1983). Introduction to the Principles of Drug Design. Elsevier. pp. 187–. ISBN 978-1-4831-8350-3. https://books.google.com/books?id=mElhDAAAQBAJ&pg=PA187.
 |


