(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description
AL-1095 Clinical data ATC code Legal status Legal status
Identifiers
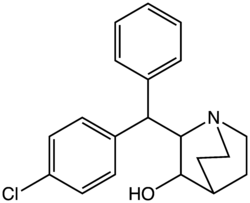
2-(1-phenyl-1-(p-chlorophenyl)methyl)-3-hydroxyquinuclidine
CAS Number PubChem CID ChemSpider UNII Chemical and physical data Formula C 20 H 22 Cl N O Molar mass −1 3D model (JSmol )
OC(C1CCN2CC1)C2C(C3=CC=C(Cl)C=C3)C4=CC=CC=C4
InChI=1S/C20H22ClNO/c21-17-8-6-15(7-9-17)18(14-4-2-1-3-5-14)19-20(23)16-10-12-22(19)13-11-16/h1-9,16,18-20,23H,10-13H2/t18?,19-,20+/m0/s1
Y Key:JXCMZYHEZWCLOD-NRRUETGQSA-N
Y N Y (what is this?) (verify)
AL-1095 ,[1] stimulant drug with comparable effects to amphetamine ,[2] [3]
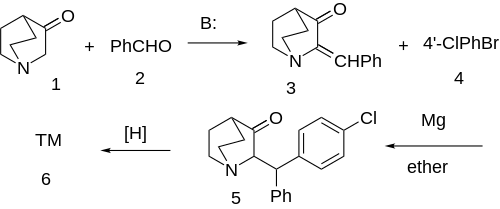
Synthesis The first-step is a mixed-aldol condensation between 3-quinuclidinone [3731-38-2] (1 ) and benzaldehyde (2 ) gives 2-benzylidene-3-oxoquinuclidine [24123-89-5] (3 ). The conjugate addition of the Grignard reagent formed from 4-bromochlorobenzene [106-39-8] (4 ) to the enone gives the benzhydryl (5 ). MPV reduction of the carbonyl gives the syn stereoisomers, whereas borohydride gave trans. Both diastereoisomers are active but in only one of the enantiomers.
See also References
↑ Warawa ED, Mueller NJ, "2-(4'Halo)-Benzhydryl-3-Quinuclidinols", US patent 3506673 , issued 14 April 1970, assigned to Aldrich Chemical Company
↑ The Speed Culture: Amphetamine Use and Abuse in America 50 . ISBN 0-674-83192-6 . https://archive.org/details/speedcultureamph0000grin_n3i0 . ↑ "Quinuclidine Chemistry. 3. β-cis -2-(4'-Chlorobenzhydryl)-3-quinuclidinol, a New Central Nervous System Stimulant. Importance of the Benzhydryl Configuration". Journal of Medicinal Chemistry 18 (1): 71–4. January 1975. doi :10.1021/jm00235a016 . PMID 803245 . ↑ Warawa, E., Mueller, N., & Gylys, J. (1975). Additions and Corrections - Quinuclidine Chemistry. 3. β-cis-2-(4’-Chlorobenzhydryl)-3-quinuclidinol, a New Central Nervous System Stimulant. Importance of the Benzhydryl Configuration. Journal of Medicinal Chemistry, 18(12), 1275–1275. https://doi.org/10.1021/jm00246a600 .
Adamantanes Adenosine antagonists Alkylamines Ampakines Arylcyclohexylamines Benzazepines Cholinergics Convulsants Eugeroics Oxazolines Phenethylamines
1-(4-Methylphenyl)-2-aminobutane 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane 2-Fuoroamphetamine 2-Fuoromethamphetamine 2-OH-PEA 2-Phenyl-3-aminobutane 2,3-MDA 3-Fuoroamphetamine 3-Fluoroethamphetamine 3-Fluoromethcathinone 3-Methoxyamphetamine 3-Methylamphetamine 3,4-DMMC 4-BMC 4-CMC 4-Fluoroamphetamine 4-Fluoromethamphetamine 4-MA 4-Methylbuphedrone 4-Methylcathinone 4-MEAP 4-MMA 4-Methylpentedrone 4-MTA 6-FNE AL-1095 Alfetamine a-Ethylphenethylamine Amfecloral Amfepentorex Amfepramone Amidephrine 2-Amino-1,2-dihydronaphthalene 2-Aminoindane 5-(2-Aminopropyl)indole 2-Aminotetralin Acridorex Amphetamine (Dextroamphetamine , Levoamphetamine )Amphetaminil Arbutamine β-Methylphenethylamine β-Phenylmethamphetamine Benfluorex Benzedrone Benzphetamine BDB BOH 3-Benzhydrylmorpholine BPAP Buphedrone Bupropion Butylone Camfetamine Cathine Cathinone Chlorphentermine Cilobamine Cinnamedrine Clenbuterol Clobenzorex Cloforex Clortermine Cypenamine D -DeprenylDenopamine Dimethoxyamphetamine Dimethylamphetamine Dimethylcathinone Dobutamine DOPA (Dextrodopa , Levodopa )Dopamine Dopexamine Droxidopa EBDB Ephedrine Epinephrine Epinine Etafedrine Ethcathinone Ethylnorepinephrine Ethylone Etilamfetamine Etilefrine Famprofazone Fencamfamin Fencamine Fenethylline Fenfluramine (Dexfenfluramine , Levofenfluramine )Fenproporex Feprosidnine Flephedrone Fludorex Formetorex Furfenorex Gepefrine Hexapradol Hexedrone HMMA Hordenine 4-Hydroxyamphetamine 5-Iodo-2-aminoindane Ibopamine Indanylamphetamine Iofetamine Isoetarine Isoethcathinone Isoprenaline L -DeprenylLefetamine Lisdexamfetamine Lophophine MBDB MDA (tenamfetamine) MDBU MDEA MDMA (midomafetamine) MDMPEA MDOH MDPR MDPEA Mefenorex Mephedrone Mephentermine Metanephrine Metaraminol Mesocarb Methamphetamine (Dextromethamphetamine , Levomethamphetamine )Methoxamine Methoxyphenamine MMA Methcathinone Methedrone Methoxyphenamine Methylenedioxycathinone Methylone Mexedrone MMDA MMDMA MMMA Morforex N,alpha-Diethylphenylethylamine N-Ethylbuphedrone N-Ethylhexedrone N,N-Dimethylphenethylamine Naphthylamphetamine Nisoxetine Norepinephrine Norfenefrine Norfenfluramine Normetanephrine L -NorpseudoephedrineOctopamine (drug) Orciprenaline Ortetamine Oxifentorex Oxilofrine PBA PCA PCMA PHA Pentorex Pentedrone Pentylone Phenatine Phenpromethamine Phentermine Phenylalanine Phenylephrine Phenylpropanolamine Pholedrine PIA PMA PMEA PMMA PPAP Phthalimidopropiophenone Prenylamine Propylamphetamine Pseudoephedrine Ropinirole Salbutamol (Levosalbutamol )Sibutramine Solriamfetol Synephrine Theodrenaline Tiflorex Tranylcypromine Tyramine Tyrosine Xylopropamine Zylofuramine Phenylmorpholines Piperazines Piperidines Pyrrolidines Racetams Tropanes Tryptamines Others
Original source: https://en.wikipedia.org/wiki/AL-1095. Read more