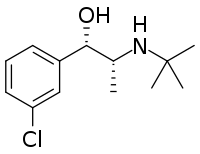
Chemistry:Erythrohydrobupropion
 | |
| Clinical data | |
|---|---|
| Other names | erythro-Hydrobupropion; Erythrohydroxybupropion; BW 287; BW 17U; erythro-3-Chloro-N-tert-butyl-β-hydroxy-α-methylphenethylamine; erythro-3-Chloro-N-tert-butyl-β-hydroxyamphetamine |
| Pharmacokinetic data | |
| Metabolism | Hydroxylation (CYP2B6, CYP2C19), glucuronidation (UGTs)[1] |
| Elimination half-life | 33 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H20ClNO |
| Molar mass | 241.76 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Erythrohydrobupropion (developmental code names BW 287, BW 17U) is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a minor active metabolite of the antidepressant drug bupropion (Wellbutrin).[1][2] Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities.[1] Erythrohydrobupropion exists as two isomers, (1R,2S)-erythrohydrobupropion and (1S,2R)-erythrohydrobupropion.[3][1] Other metabolites of bupropion include hydroxybupropion and threohydrobupropion.[1][2]
Information on the pharmacological actions of erythrohydrobupropion is scarce.[1] In any case, it is about 20% as pharmacologically potent as bupropion and in the range of 20 to 50% as potent as bupropion in mouse models of depression.[1][2] It circulates at similar concentrations as bupropion during bupropion therapy.[1][2] Conversely, two other metabolites, hydroxybupropion and threohydrobupropion, circulate at higher concentrations than bupropion.[1][2]
Erythrohydrobupropion is formed from bupropion via reduction of the ketone group primarily by 11β-hydroxysteroid dehydrogenase-1 and to a minor extent by aldo-keto reductases.[1] It can also be formed from bupropion by carbonyl reductases.[1][2] The compound is metabolized by the cytochrome P450 enzymes CYP2B6 and CYP2C19 into erythro-4'-hydroxy-hydrobupropion and by various glucuronosyltransferase enzymes into glucuronide conjugates.[1] The elimination half-life of erythrohydrobupropion is approximately 33 hours.[1][2] Its half-life may be longer in older people.[2]
Insomnia during bupropion therapy has been associated with erythrohydrobupropion concentrations.[1] Administration of erythrohydrobupropion in mice produces seizures at sufficiently high doses similarly to bupropion and other metabolites.[1] Erythrohydrobupropion is a CYP2D6 inhibitor and accounts for about 9% of CYP2D6 inhibition during bupropion therapy, with hydroxybupropion accounting for 65% and threohydrobupropion accounting for 21%.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 "Pharmacokinetic and pharmacodynamic of bupropion: integrative overview of relevant clinical and forensic aspects". Drug Metab Rev 51 (3): 293–313. August 2019. doi:10.1080/03602532.2019.1620763. PMID 31124380.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations". Clin Ther 27 (11): 1685–95. November 2005. doi:10.1016/j.clinthera.2005.11.011. PMID 16368442.
- ↑ "Chiral Plasma Pharmacokinetics and Urinary Excretion of Bupropion and Metabolites in Healthy Volunteers". J Pharmacol Exp Ther 358 (2): 230–8. August 2016. doi:10.1124/jpet.116.232876. PMID 27255113.
 |
