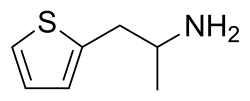
Chemistry:Thiopropamine
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C7H11NS |
| Molar mass | 141.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Thiopropamine is a stimulant drug which is an analogue of amphetamine where the phenyl ring has been replaced by thiophene. It has similar stimulant effects to amphetamine but with around one third the potency. The N-methyl and thiophen-3-yl analogues are also known and are somewhat more potent, though still generally weaker than the corresponding amphetamines.[1][2]
Pharmacology
Like amphetamine and most of its analogues, thiopropamine most likely is a norepinephrine–dopamine reuptake inhibitor and/or releasing agent.[citation needed]
Thiopropamine is likely to be metabolized into active 4-hydroxymethiopropamine and thiophene S-oxides.[3][4] These are further deaminated by CYP2C in liver transforming them into inactive 1-(Thiophen-2-yl)-2-propan-2-one which is a phenylacetone derivative.[5] Propan-2-amines are not metabolized by monoamine oxidases and most actually behave as competitive monoamine oxidase inhibitors.[6][7]
References
- ↑ "Comparative Physiological Actions of Phenyl-, Thienyl- and Furylisopropylamines". Journal of Pharmacology and Experimental Therapeutics 72 (3): 265–75. July 1941. http://jpet.aspetjournals.org/content/72/3/265.abstract.
- ↑ "3-Substituted Thiophenes. VIII. 3-Thienylalkylamines". Journal of the American Chemical Society 76 (17): 4466–4468. September 1954. doi:10.1021/ja01646a054.
- ↑ "Chemical and Biological Oxidation of Thiophene: Preparation and Complete Characterization of Thiophene S-Oxide Dimers and Evidence for Thiophene S-Oxide as an Intermediate in Thiophene Metabolism in Vivo and in Vitro". Journal of the American Chemical Society 119 (7): 1565–1571. February 1997. doi:10.1021/ja962466g.
- ↑ "Evidence for thiophene-S-oxide as a primary reactive metabolite of thiophene in vivo: formation of a dihydrothiophene sulfoxide mercapturic acid". Biochemical and Biophysical Research Communications 186 (3): 1624–30. August 1992. doi:10.1016/S0006-291X(05)81594-3. PMID 1510686.
- ↑ "Deamination of amphetamines by cytochromes P450: studies on substrate specificity and regioselectivity with microsomes and purified CYP2C subfamily isozymes". The Journal of Toxicological Sciences 22 (1): 65–73. February 1997. doi:10.2131/jts.22.65. PMID 9076658.
- ↑ "Inhibition of monoamine oxidase by amphetamine and related compounds". Biochemical Pharmacology 25 (18): 2073–7. September 1976. doi:10.1016/0006-2952(76)90432-9. PMID 985546.
- ↑ "In vivo monoamine oxidase inhibition by d-amphetamine". Biochemical Pharmacology 29 (10): 1347–54. May 1980. doi:10.1016/0006-2952(80)90429-3. PMID 6901611.
 |

