Chemistry:Metaphit
| |
| Names | |
|---|---|
| Preferred IUPAC name
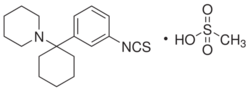
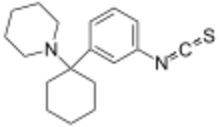
1-[1-(3-Isothiocyanatophenyl)cyclohexyl]piperidine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H24N2S | |
| Molar mass | 300.462 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metaphit (1-[1-(3-Isothiocyanato)phenyl]cyclohexylpiperidine) is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the m-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly (forming a covalent bond) to the PCP binding site on the NMDA receptor complex.[1] However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex.[2] Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens.[3] Metaphit was the first acylating ligand used to study the cocaine receptor.[4] It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP.[5]
References
- ↑ Rafferty, Michael F.; Mattson, Mariena; Jacobson, Arthur E.; Rice, Kenner C. (1985). "A specific acylating agent for the [3H]phencyclidine receptors in rat brain". FEBS Letters 181 (2): 318–22. doi:10.1016/0014-5793(85)80284-2. PMID 2982662.
- ↑ Danysz, Wojciech (1991). "Metaphit fails to antagonize PCP-induced passive avoidance deficit". Pharmacology Biochemistry and Behavior 38 (1): 231–3. doi:10.1016/0091-3057(91)90618-C. PMID 1826788.
- ↑ French, Edward D.; Jacobson, Arthur E.; Rice, Kenner C. (1987). "Metaphit, a proposed phencyclidine (PCP) antagonist, prevents PCP-induced locomotor behavior through mechanisms unrelated to specific blockade of PCP receptors". European Journal of Pharmacology 140 (3): 267–74. doi:10.1016/0014-2999(87)90283-4. PMID 2820762.
- ↑ Carroll, F. Ivy; Lewin, Anita H.; Boja, John W.; Kuharf, Michael J. (1992). "Cocaine receptor: Biochemical characterization and structure-activity relationships of cocaine analogs at the dopamine transporter". Journal of Medicinal Chemistry 35 (6): 969–81. doi:10.1021/jm00084a001. PMID 1552510.
- ↑ Schweri, MM; Thurkauf, A; Mattson, MV; Rice, KC. "Fourphit: a selective probe for the methylphenidate binding site on the dopamine transporter". J Pharmacol Exp Ther 261: 936-42. PMID 1602399.
 |