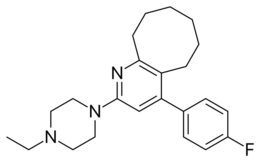
Chemistry:Blonanserin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lonasen |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 55%[1] |
| Metabolism | CYP3A4[1] |
| Elimination half-life | 12 h[1] |
| Excretion | 59% (urine), 30% (faeces)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H30FN3 |
| Molar mass | 367.512 g·mol−1 |
| 3D model (JSmol) | |
| |
Blonanserin, sold under the brand name Lonasen, is a relatively new atypical antipsychotic (approved by PMDA in January 2008)[2] commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia.[3] Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension.[4] As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.[5]
Medical uses
Blonanserin is used to treat schizophrenia in Japan and South Korea but not in the US.[6]
Adverse effects
As with many of the atypical antipsychotics, blonanserin can elicit cardio metabolic risks. While the side effects of blonanserin – such as weight gain, cholesterol and triglyceride levels, glucose levels and other blood lipid levels – do not differ greatly from other atypical antipsychotics, the specificity of blonanserin appears to elicit milder side effects, with less weight gain in particular.[5]
Pharmacology
Pharmacodynamics
Blonanserin acts as a mixed 5-HT2A (Ki = 0.812 nM) and D2 receptor (Ki = 0.142 nM) antagonist and also exerts some blockade of α1-adrenergic receptors (Ki = 26.7 nM).[7][8] Blonanserin also shows significant affinity for the D3 receptor (Ki = 0.494 nM).[9] It lacks significant affinity for numerous other sites including the 5-HT1A, 5-HT3, D1, α2-adrenergic, β-adrenergic, H1, and mACh receptors and the monoamine transporters,[8] though it does possess low affinity for the sigma receptor (IC50 = 286 nM).[8]
Blonanserin has a relatively high affinity towards the 5-HT6 receptor perhaps underpinning its recently unveiled efficacy in treating the cognitive symptoms of schizophrenia.[7][10] The efficacy of blonanserin can in part be attributed to its chemical structure, which is unique from those of other atypical antipsychotics.[11] Specifically, the addition of hydroxyl groups to blonanserin's unique eight membered ring results in the (R) stereoisomer of the compound demonstrating increased affinity for the indicated targets.[12]
| Receptor | Ki [nM] (Blonanserin)* [7] | Ki [nM] (N-deethylblonanserin)* [3] |
|---|---|---|
| D1 | 1070 | 1020 |
| D2 | 0.142 | 1.38 |
| D3 | 0.494 | 0.23 |
| D4 | 150 | - |
| D5 | 2600 | - |
| 5-HT1A | 804 | - |
| 5-HT2A | 0.812 | 1.28 |
| 5-HT2C | 26.4 | 4.50 |
| 5-HT6 | 11.7 | 5.03 |
| 5-HT7 | 183 | - |
| α1 | 26.7 (Rat brain) | 206 (Rat receptor) |
| α2 | 530 (Rat cloned) | - |
| M1 | 100 | - |
| H1 | 765 | - |
* Towards human receptors unless otherwise specified.
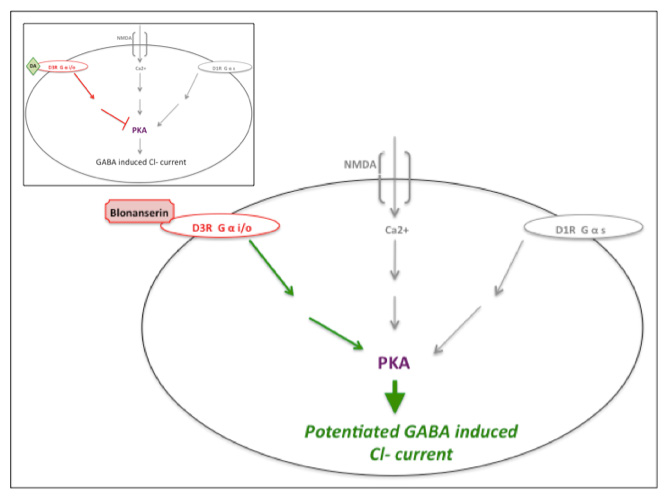
Action at the Dopamine-D3 receptor
Blonanserin has antagonistic action at dopamine-D3 receptors that potentiates phosphorylation levels of Protein kinase A (PKA) and counteracts decreased activity at the dopamine-D1 and/or NMDA receptors, thus potentiating GABA induced Cl- currents.[9][13] Olanzapine does not appear to affect PKA activity.[9][14] Many antipsychotics, such as haloperidol, chlorpromazine, risperidone and olanzapine primarily antagonize serotonin 5-HT2A and dopamine-D2 receptors and lack known action at dopamine-D2/3 receptors.[9][11]
|
| Blonanserin action at dopamine-D3 receptor. Cartoon of blonanserin's antagonistic impact at the dopamine-D3 receptor, reversing inhibition of PKA activity (also regulated by dopamine-D1 and NMDA activity) thus potentiating GABA induced Cl- current. Inset illustrates uninterrupted dopamine (DA) activity at the dopamine-D3 receptor. Inspired by Hida et al. (2014) and Yokota et al. (2002).[9][13] |
Pharmacokinetics
Blonanserin is administered 4 mg orally twice a day or 8 mg once a day, for an adult male with a body mass index between 19–24 kg/m2 and a body weight equal to or greater than 50 kg.[15] The drug is absorbed by a two compartment (central and peripheral) model with first-order absorption and elimination.[1] The half-life of blonanserin is dependent on the dose. A single dose of 4 mg has a half-life of 7.7 ± 4.63 h and a single dose of 8 mg has a half-life of 11.9 ± 4.3 h.[15] The increase of half-life with dose is possibly attributed to there being more individual concentration per time points below the lower limit necessary for quantification in the lower single dose.[15]
Blonanserin is not a charged compound and exhibits very little chemical polarity. The polar surface area of Blonanserin is 19.7 Å[16] It is commonly accepted that a compound needs to have polar surface area less than 90 Å to cross the blood brain barrier so blonanserin is expected to be quite permeable as is demonstrated by a high brain/ plasma ratio of 3.88.[17]
Due to the good permeability of blonanserin, the volume of distribution in the central nervous system is greater than that in the periphery (Vd central = 9500 L, Vd periphery = 8650 L) although it is slower to absorb into the central compartment.[1]
Blonanserin does not meet the criteria in Lipinski's rule of five.[16]
Effects of food intake
Food intake slows the absorption of blonanserin and increases the bioavailability peripherally relative to centrally.[1] Single fasting doses are safe and the effects of feeding intake are possibly explained by an interaction between blonanserin and Cytochrome P450 3A4 in the gut.[15]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Population pharmacokinetics of blonanserin in Chinese healthy volunteers and the effect of the food intake". Human Psychopharmacology 28 (2): 134–141. March 2013. doi:10.1002/hup.2290. PMID 23417765.
- ↑ "FY2007 List of Approved Products: New Drugs". Tokyo, Japan: Pharmaceuticals and Medical Devices Agency. http://www.pmda.go.jp/english/service/pdf/list/NewdrugsFY2007.pdf.
- ↑ 3.0 3.1 "Blonanserin: a review of its use in the management of schizophrenia". CNS Drugs 24 (1): 65–84. January 2010. doi:10.2165/11202620-000000000-00000. PMID 20030420.
- ↑ "AD-5423 Dainippon Pharmaceutical Co Ltd". IDrugs 1 (7): 813–817. November 1998. PMID 18465651.
- ↑ 5.0 5.1 "Blonanserin for schizophrenia: systematic review and meta-analysis of double-blind, randomized, controlled trials". Journal of Psychiatric Research 47 (2): 149–154. February 2013. doi:10.1016/j.jpsychires.2012.10.011. PMID 23131856.
- ↑ "Asenapine, blonanserin, iloperidone, lurasidone, and sertindole: distinctive clinical characteristics of 5 novel atypical antipsychotics". Clinical Neuropharmacology 36 (6): 223–238. 2013. doi:10.1097/wnf.0b013e3182aa38c4. PMID 24201235.
- ↑ 7.0 7.1 7.2 "Profile of blonanserin for the treatment of schizophrenia". Neuropsychiatric Disease and Treatment 9: 587–594. 2013. doi:10.2147/NDT.S34433. PMID 23766647.
- ↑ 8.0 8.1 8.2 "Pharmacological profile of AD-5423, a novel antipsychotic with both potent dopamine-D2 and serotonin-S2 antagonist properties". The Journal of Pharmacology and Experimental Therapeutics 264 (1): 158–165. January 1993. PMID 8093723. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=8093723.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Blonanserin ameliorates phencyclidine-induced visual-recognition memory deficits: the complex mechanism of blonanserin action involving D₃-5-HT₂A and D₁-NMDA receptors in the mPFC". Neuropsychopharmacology 40 (3): 601–613. February 2015. doi:10.1038/npp.2014.207. PMID 25120077.
- ↑ "Effect of blonanserin on cognitive function in antipsychotic-naïve first-episode schizophrenia". Human Psychopharmacology 27 (1): 90–100. January 2012. doi:10.1002/hup.1276. PMID 22278973.
- ↑ 11.0 11.1 "Crystal structure of an antipsychotic agent, 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine (blonanserin)". Analytical Sciences 18 (11): 1289–1290. November 2002. doi:10.2116/analsci.18.1289. PMID 12458724.
- ↑ "Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent". Bioorganic & Medicinal Chemistry Letters 15 (4): 1055–1059. February 2005. doi:10.1016/j.bmcl.2004.12.028. PMID 15686911.
- ↑ 13.0 13.1 "The effects of neuroleptics on the GABA-induced Cl- current in rat dorsal root ganglion neurons: differences between some neuroleptics". British Journal of Pharmacology 135 (6): 1547–1555. March 2002. doi:10.1038/sj.bjp.0704608. PMID 11906969.
- ↑ "Effect of AD-5423 on animal models of schizophrenia: phencyclidine-induced behavioral changes in mice". NeuroReport 14 (2): 269–272. February 2003. doi:10.1097/00001756-200302100-00023. PMID 12598744.
- ↑ 15.0 15.1 15.2 15.3 "The pharmacokinetic and safety profiles of blonanserin in healthy Chinese volunteers after single fasting doses and single and multiple postprandial doses". Clinical Drug Investigation 34 (3): 213–222. March 2014. doi:10.1007/s40261-013-0167-9. PMID 24399453.
- ↑ 16.0 16.1 "Blonanserin". PubMed. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Blonanserin.
- ↑ "Striatal and extrastriatal dopamine D2 receptor occupancy by a novel antipsychotic, blonanserin: a PET study with [11C]raclopride and [11C]FLB 457 in schizophrenia". Journal of Clinical Psychopharmacology 33 (2): 162–169. April 2013. doi:10.1097/jcp.0b013e3182825bce. PMID 23422369.
 |

