Chemistry:Escitalopram
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛsəˈtæləˌpræm/ |
| Trade names | Cipralex, Lexapro, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603005 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Selective serotonin reuptake inhibitor (SSRI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | ~56% |
| Metabolism | Liver, specifically the enzymes CYP3A4 and CYP2C19 |
| Metabolites | desmethylcitalopram, didesmethylcitalopram |
| Elimination half-life | 27–32 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
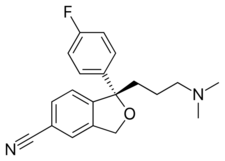
| Formula | C20H21FN2O |
| Molar mass | 324.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Escitalopram, sold under the brand names Lexapro and Cipralex, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[4] Escitalopram is mainly used to treat major depressive disorder and generalized anxiety disorder.[4] It is taken by mouth,[4] available commercially as an oxalate salt exclusively.
Common side effects include trouble sleeping, nausea, sexual problems, and feeling tired.[4] More serious side effects may include suicidal thoughts in people up to the age of 24 years.[4] It is unclear if use during pregnancy or breastfeeding is safe.[5] Escitalopram is the (S)-enantiomer of citalopram (which exists as a racemate), hence the name es-citalopram.[4]
Escitalopram was approved for medical use in the United States in 2002.[4] Escitalopram is rarely replaced by twice the dose of citalopram, though escitalopram is safer and more effective.[6] It is on the World Health Organization's List of Essential Medicines.[7] In 2021, it was the fifteenth most commonly prescribed medication in the United States, with more than 30 million prescriptions.[8][9]
Medical uses
Escitalopram has FDA approval for the treatment of major depressive disorder in adolescents and adults, and generalized anxiety disorder in adults.[4] In European countries and the United Kingdom, it is approved for depression (MDD) and anxiety disorders; these include: general anxiety disorder (GAD), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and panic disorder with or without agoraphobia. In Australia it is approved for major depressive disorder.[10][11][12]
Depression
Escitalopram is among the most effective and well-tolerated antidepressants for the short-term (acute) treatment of major depressive disorder in adults.[13][14] It is also the safest one to give to children and adolescents.[15][16]
Controversy existed regarding the effectiveness of escitalopram compared with its predecessor, citalopram. The importance of this issue followed from the greater cost of escitalopram relative to the generic mixture of isomers of citalopram, prior to the expiration of the escitalopram patent in 2012, which led to charges of evergreening. Accordingly, this issue has been examined in at least 10 different systematic reviews and meta analyses. (As of 2012), reviews had concluded (with caveats in some cases) that escitalopram is modestly superior to citalopram in efficacy and tolerability.[17][18][19][20]
Anxiety disorders
Escitalopram appears to be effective in treating generalized anxiety disorder, with relapse on escitalopram at 20% rather than placebo at 50%, which translates to a number needed to treat of 3.33.[21][22] Escitalopram appears effective in treating social anxiety disorder as well.[23]
Other
Escitalopram is effective in reducing the symptoms of premenstrual syndrome, whether taken continuously or in the luteal phase only.[24]
Side effects
Escitalopram, like other SSRIs, has been shown to affect sexual function, causing side effects such as decreased libido, delayed ejaculation, and anorgasmia.[25][26]
There is also evidence that SSRIs may cause an increase in suicidal ideation. An analysis conducted by the FDA found a statistically insignificant 1.5 to 2.4-fold (depending on the statistical technique used) increase of suicidality among the adults treated with escitalopram for psychiatric indications.[27][28][29] The authors of a related study note the general problem with statistical approaches: due to the rarity of suicidal events in clinical trials, it is hard to draw firm conclusions with a sample smaller than two million patients.[30]
Citalopram and escitalopram are associated with dose-dependent QT interval prolongation[31] and should not be used in those with congenital long QT syndrome or known pre-existing QT interval prolongation, or in combination with other medicines that prolong the QT interval. ECG measurements should be considered for patients with cardiac disease, and electrolyte disturbances should be corrected before starting treatment. In December 2011, the UK implemented new restrictions on the maximum daily doses at 20 mg for adults and 10 mg for those older than 65 years or with liver impairment.[32][33] There are concerns of higher rates of QT prolongation and torsades de pointes compared with other SSRIs.[34][35] The US Food and Drug Administration and Health Canada did not similarly order restrictions on escitalopram dosage, only on its predecessor citalopram.[36]
Very common effects
Very common effects (>10% incidence) include:[37][38][39][2][40]
- Headache (24%)
- Nausea (18%)
- Ejaculation disorder (9–14%)
- Somnolence (4–13%)
- Insomnia (7–12%)
Common (1–10% incidence)
Common effects (1–10% incidence) include:
- Abnormal dreams
- Anisocoria
- Anorgasmia
- Anxiety
- Arthralgia (joint pain)
- Constipation
- Decreased or increased appetite
- Diarrhea
- Dilated pupils
- Dizziness
- Dry mouth
- Excessive sweating
- Fatigue
- Impotence (erectile dysfunction)
- Libido changes
- Myalgia (muscular aches and pains)
- Paraesthesia (abnormal skin sensation)
- Pyrexia (fever)
- Restlessness
- Sinusitis (nasal congestion)
- Tremor
- Vomiting
- Yawning
Psychomotor effects
The most common effect is fatigue or somnolence, particularly in older adults,[41] although patients with pre-existing daytime sleepiness and fatigue may experience paradoxical improvement of these symptoms.[42] Escitalopram has not been shown to affect serial reaction time, logical reasoning, serial subtraction, multitask, or Mackworth Clock task performance.[43]
Discontinuation symptoms
Escitalopram discontinuation, particularly abruptly, may cause certain withdrawal symptoms such as "electric shock" sensations,[44] colloquially called "brain shivers" or "brain zaps" by those affected. Frequent symptoms in one study were dizziness (44%), muscle tension (44%), chills (44%), confusion or trouble concentrating (40%), amnesia (28%), and crying (28%). Very slow tapering was recommended.[45] There have been spontaneous reports of discontinuation of Lexapro and other SSRIs and SNRIs, especially when abrupt, leading to dysphoric mood, irritability, agitation, anxiety, headache, lethargy, emotional lability, insomnia, and hypomania. Other symptoms such as panic attacks, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), mania, worsening of depression, and suicidal ideation can emerge when the dose is adjusted down.[46]
Sexual dysfunction
Some people experience persistent sexual side effects when taking SSRIs or after discontinuing them.[47] Symptoms of medication-induced sexual dysfunction from antidepressants include difficulty with orgasm, erection, or ejaculation.[47] Other symptoms may be genital anesthesia, anhedonia, decreased libido, vaginal lubrication issues, and nipple insensitivity in women. Rates are unknown, and there is no established treatment.[48]
Pregnancy
Antidepressant exposure (including escitalopram) is associated with shorter duration of pregnancy (by three days), increased risk of preterm delivery (by 55%), lower birth weight (by 75 g), and lower Apgar scores (by <0.4 points). Antidepressant exposure is not associated with an increased risk of spontaneous abortion.[49] There is a tentative association of SSRI use during pregnancy with heart problems in the baby.[50] The advantages of their use during pregnancy may thus outweigh the possible negative effects on the baby.[50]
Overdose
Excessive doses of escitalopram usually cause relatively minor untoward effects, such as agitation and tachycardia. However, dyskinesia, hypertonia, and clonus may occur in some cases. Therapeutic blood levels of escitalopram are usually in the range of 20–80 μg/L but may reach 80–200 μg/L in the elderly, patients with hepatic dysfunction, those who are poor CYP2C19 metabolizers or following acute overdose. Monitoring of the drug in plasma or serum is generally accomplished using chromatographic methods. Chiral techniques are available to distinguish escitalopram from its racemate, citalopram.[33][51][52]
Pharmacology
Mechanism of action
| Site | Ki (nM) |
|---|---|
| SERT | 0.8–1.1 |
| NET | 7,800 |
| DAT | 27,400 |
| 5-HT1A | >1,000 |
| 5-HT2A | >1,000 |
| 5-HT2C | 2,500 |
| α1 | 3,900 |
| α2 | >1,000 |
| D2 | >1,000 |
| H1 | 2,000 |
| mACh | 1,240 |
| hERG | 2,600 (IC50) |
Escitalopram increases intrasynaptic levels of the neurotransmitter serotonin by blocking the reuptake of the neurotransmitter into the presynaptic neuron. Over time, this leads to a downregulation of pre-synaptic 5-HT1A receptors, which is associated with an improvement in passive stress tolerance, and delayed downstream increase in expression of brain-derived neurotrophic factor, which may contribute to a reduction in negative affective biases.[55][56]
Of the SSRIs currently available, escitalopram has the highest selectivity for the serotonin transporter (SERT) compared to the norepinephrine transporter (NET), making the side-effect profile relatively mild in comparison to less-selective SSRIs.[57]
Escitalopram is a substrate of P-glycoprotein and hence P-glycoprotein inhibitors such as verapamil and quinidine may improve its blood brain barrier penetrability.[58] In a preclinical study in rats combining escitalopram with a P-glycoprotein inhibitor, its antidepressant-like effects were enhanced.[58]
Interactions
Escitalopram, similarly to other SSRIs, may increase bleed risk with NSAIDs (ibuprofen, naproxen, mefenamic acid), antiplatelet drugs, anticoagulants, omega-3 fatty acids, vitamin E, and garlic supplements due to escitalopram's inhibitory effects on platelet aggregation via blocking serotonin transporters on platelets.[59] Escitalopram inhibits CYP2D6, and hence may increase plasma levels of a number of CYP2D6 substrates such as aripiprazole, risperidone, tramadol, codeine, etc. As escitalopram is only a weak inhibitor of CYP2D6, analgesia from tramadol may not be affected.[60] Escitalopram should be taken with caution when using St. John's wort, ginseng, dextromethorphan (DXM), linezolid, tramadol, and other serotonergic drugs due to the risk of serotonin syndrome.[61][62] Exposure to escitalopram is increased moderately, by about 50%, when it is taken with omeprazole. The authors of this study suggested that this increase is unlikely to be of clinical concern.[63]
Bupropion has been found to significantly increase citalopram plasma concentration and systemic exposure; (As of April 2018) the interaction with escitalopram had not been studied, but some monographs warned of the potential interaction.[64]
Escitalopram can also prolong the QT interval, and hence it is not recommended in patients that are concurrently on other medications that also have the ability to prolong the QT interval. These drugs include antiarrhythmics, antipsychotics, tricyclic antidepressants, some antihistamines (astemizole, mizolastine), macrolide and fluoroquinolone antibiotics, some 5-HT3 receptor antagonists (except palonosetron), and some antiretrovirals (ritonavir, saquinavir, lopinavir).[32] As an SSRI, escitalopram should not be given concurrently with MAOIs.[57]
Chemistry
Escitalopram is the (S)-enantiomer (left-handed version) of the racemate citalopram, which is responsible for its name: escitalopram.[4] [65]
History
Escitalopram was developed in cooperation between Lundbeck and Forest Laboratories. Its development was initiated in 1997, and the resulting new drug application was submitted to the US FDA in March 2001. The short time (3.5 years) it took to develop escitalopram can be attributed to the previous experience of Lundbeck and Forest with citalopram, which has similar pharmacology.[66]
Society and culture
Legal status
The FDA issued the approval of escitalopram for major depression in August 2002, and for generalized anxiety disorder in December 2003. In May 2006, the FDA approved a generic version of escitalopram by Teva.[67] In July 2006, the U.S. District Court of Delaware decided in favor of Lundbeck regarding a patent infringement dispute and ruled the patent on escitalopram valid.[68]
In 2006, Forest Laboratories was granted an 828-day (2 years and 3 months) extension on its US patent for escitalopram.[69] This pushed the patent expiration date from 7 December 2009, to 14 September 2011. Together with the 6-month pediatric exclusivity, the final expiration date was 14 March 2012.
Allegations of illegal marketing
In 2004, separate civil suits alleging illegal marketing of citalopram and escitalopram for use by children and teenagers by Forest were initiated by two whistleblowers: a physician named Joseph Piacentile and a Forest salesman named Christopher Gobble.[70] In February 2009, the suits were joined. Eleven states and the District of Columbia filed notices of intent to intervene as plaintiffs in the action.
The suits alleged that Forest illegally engaged in off-label promotion of Lexapro for use in children; hid the results of a study showing lack of effectiveness in children; paid kickbacks to physicians to induce them to prescribe Lexapro to children; and conducted so-called "seeding studies" that were, in reality, marketing efforts to promote the drug's use by doctors.[71][72] Forest denied the allegations[73] but ultimately agreed to settle with the plaintiffs for over $313 million.[74]
Brand names
Escitalopram is sold under many brand names worldwide such as Cipralex, Lexapro, Lexam, Mozarin, Aciprex, Depralin, Ecytara, Elicea, Gatosil, Nexpram, Nexito, Nescital, Szetalo, Stalopam, Pramatis, Betesda, Scippa and Rexipra.[1][75]
References
- ↑ 1.0 1.1 drugs.com Drugs.com international: Escitalopram Page accessed 25 April 2015
- ↑ 2.0 2.1 "Lexapro- escitalopram tablet, film coated; Lexapro- escitalopram solution". 17 November 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a.
- ↑ Human Medicines Division (September 2022). "Active substance(s): escitalopram". List of nationally authorised medicinal products. European Medicines Agency. https://www.ema.europa.eu/documents/psusa/escitalopram-list-nationally-authorised-medicinal-products-psusa/00001265/202112_en.pdf.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 "X". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/escitalopram-oxalate.html.
- ↑ "Escitalopram (Lexapro) Use During Pregnancy". https://www.drugs.com/pregnancy/escitalopram.html.
- ↑ "Protocol for switching patients from escitalopram to citalopram". 2015. http://www.ipswichandeastsuffolkccg.nhs.uk/LinkClick.aspx?fileticket=6JoKJA8nPsg%3D.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Escitalopram - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Escitalopram.
- ↑ "Escitalopram oxalate" (in en). Australian Prescriber 26: 146–151. 2003. doi:10.18773/austprescr.2003.107. https://www.nps.org.au/australian-prescriber/articles/escitalopram-oxalate. Retrieved 15 March 2020.
- ↑ "Lundbeck's Cipralex gets EU ok for OCD treatment" (in en). Reuters. 12 January 2007. https://www.reuters.com/article/lundbeck-idUSDKT00159920070112.
- ↑ "Cipralex 10 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc)". https://www.medicines.org.uk/emc/product/7718/smpc.
- ↑ "The most effective antidepressants for adults revealed in major review". NIHR Evidence (National Institute for Health and Care Research). 3 April 2018. doi:10.3310/signal-00580. https://evidence.nihr.ac.uk/alert/the-most-effective-antidepressants-for-adults-revealed-in-major-review.
- ↑ "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet 391 (10128): 1357–1366. April 2018. doi:10.1016/S0140-6736(17)32802-7. PMID 29477251.
- ↑ "Psychiatric drugs given to children and adolescents have been ranked in order of safety". NIHR Evidence (National Institute for Health and Care Research). 1 September 2020. doi:10.3310/alert_40795.
- ↑ "Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects". World Psychiatry 19 (2): 214–232. June 2020. doi:10.1002/wps.20765. PMID 32394557.
- ↑ "Effectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness model". PLOS ONE 7 (8): e42003. 2012. doi:10.1371/journal.pone.0042003. PMID 22876296. Bibcode: 2012PLoSO...742003R.
- ↑ "Citalopram versus other anti-depressive agents for depression". The Cochrane Database of Systematic Reviews 7 (7): CD006534. July 2012. doi:10.1002/14651858.CD006534.pub2. PMID 22786497.
- ↑ "[Clinical efficacy and achievement of a complete remission in depression: increasing interest in treatment with escitalopram]" (in fr). L'Encéphale 38 (1): 86–96. February 2012. doi:10.1016/j.encep.2011.11.003. PMID 22381728.
- ↑ "Comparison of escitalopram vs. citalopram and venlafaxine in the treatment of major depression in Spain: clinical and economic consequences". Current Medical Research and Opinion 26 (12): 2757–2764. December 2010. doi:10.1185/03007995.2010.529430. PMID 21034375.
- ↑ "Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis" (in English). Lancet 393 (10173): 768–777. February 2019. doi:10.1016/S0140-6736(18)31793-8. PMID 30712879. https://discovery.ucl.ac.uk/id/eprint/10070219/.
- ↑ "Relapse prevention and residual symptoms: a closer analysis of placebo-controlled continuation studies with escitalopram in major depressive disorder, generalized anxiety disorder, social anxiety disorder, and obsessive-compulsive disorder". The Journal of Clinical Psychiatry 71 (2): 121–129. February 2010. doi:10.4088/JCP.08m04749blu. PMID 19961809.
- ↑ "Efficacy of escitalopram in the treatment of social anxiety disorder: A meta-analysis versus placebo". European Neuropsychopharmacology 26 (6): 1062–1069. June 2016. doi:10.1016/j.euroneuro.2016.02.013. PMID 26971233.
- ↑ "Selective serotonin reuptake inhibitors for premenstrual syndrome". The Cochrane Database of Systematic Reviews 2013 (6): CD001396. June 2013. doi:10.1002/14651858.CD001396.pub3. PMID 23744611.
- ↑ "Burden of phase-specific sexual dysfunction with SSRIs". Journal of Affective Disorders 91 (1): 27–32. March 2006. doi:10.1016/j.jad.2005.12.007. PMID 16430968.
- ↑ "Lexapro prescribing information". https://www.allergan.com/Assets/PDF/Lexapro_pi.pdf.
- ↑ "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". https://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4272s1-04-FDA.ppt.
- ↑ "Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults". FDA. 17 November 2006. pp. 11–74. https://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf.
- ↑ "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants". FDA. 17 November 2006. pp. 75–140. https://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf.
- ↑ "Suicide risk and symptom reduction in patients assigned to placebo in duloxetine and escitalopram clinical trials: analysis of the FDA summary basis of approval reports". Annals of Clinical Psychiatry 19 (1): 31–36. 2007. doi:10.1080/10401230601163550. PMID 17453659.
- ↑ "QT interval and antidepressant use: a cross sectional study of electronic health records". BMJ 346: f288. January 2013. doi:10.1136/bmj.f288. PMID 23360890.
- ↑ 32.0 32.1 "Citalopram and escitalopram: QT interval prolongation—new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings". Medicines and Healthcare products Regulatory Agency. December 2011. http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON137769.
- ↑ 33.0 33.1 "Clinical and ECG effects of escitalopram overdose". Annals of Emergency Medicine 54 (3): 404–408. September 2009. doi:10.1016/j.annemergmed.2009.04.016. PMID 19556032.
- ↑ "Towards better patient care: drugs to avoid in 2017". http://english.prescrire.org/en/81/168/52722/0/NewsDetails.aspx.
- ↑ "Drugs To Avoid 2017 PDF Download". http://english.prescrire.org/Docu/DownloadDocu/PDFs/DrugsToAvoid_2017update.pdf.
- ↑ "Escitalopram and QTc prolongation". Journal of Psychiatry & Neuroscience 38 (4): E11. July 2013. doi:10.1503/jpn.130055. PMID 23791140.
- ↑ "Lexapro (escitalopram) dosing, indications, interactions, adverse effects, and more". Medscape. https://reference.medscape.com/drug/lexapro-escitalopram-342961.
- ↑ "Cipralex 5, 10 and 20 mg film-coated tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. 2 October 2013. http://www.medicines.org.uk/emc/medicine/27012/SPC/Cipralex+5%2c+10+and+20+mg+film-coated+tablets/.
- ↑ "Escitalopram-Lupin Tablets (LUPIN AUSTRALIA PTY. LTD)" (PDF). TGA eBusiness Services. Lupin Australia Pty Ltd. 21 December 2011. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2012-PI-01532-3.
- ↑ "Unequal Sized Pupils Due to Escitalopram; Adverse Events to Dietary Supplements Causing Emergency Department Visits; Compulsive Masturbation Due to Pramipexole; Metformin-Induced Lactic Acidosis Masquerading As an Acute Myocardial Infarction". Hospital Pharmacy (Thomas Land Publishers, Inc.) 51 (5): 358–361. May 2016. doi:10.1310/hpj5105-358. PMID 27303087.
- ↑ "Escitalopram Treatment of Generalized Anxiety Disorder in Older Adults—Reply". JAMA 301 (19): 1987. 20 May 2009. doi:10.1001/jama.2009.652. ISSN 0098-7484.
- ↑ "Excessive daytime sleepiness and fatigue in depressed patients and therapeutic response of a sedating antidepressant". Journal of Affective Disorders 134 (1–3): 421–426. November 2011. doi:10.1016/j.jad.2011.04.047. PMID 21616541.
- ↑ "Alertness management in aviation operations: enhancing performance and sleep". Aviation, Space, and Environmental Medicine 77 (12): 1256–1265. December 2006. doi:10.3357/asem.1879.2006. PMID 17183922.
- ↑ "Emergence of electric shock-like sensations on escitalopram discontinuation". Journal of Clinical Psychopharmacology 28 (3): 359–360. June 2008. doi:10.1097/JCP.0b013e3181727534. PMID 18480703.
- ↑ "Characteristics of Escitalopram Discontinuation Syndrome: A Preliminary Study". Clinical Neuropharmacology 39 (3): 125–127. June 2016. doi:10.1097/WNF.0000000000000139. PMID 27171568.
- ↑ "Lexapro (Escitalopram Oxalate) Drug Information: Warnings and Precautions - Prescribing Information at RxList". http://www.rxlist.com/cgi/generic/lexapro_wcp.htm.
- ↑ 47.0 47.1 American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. pp. 446–449. ISBN 9780890425558.
- ↑ "Post-SSRI Sexual Dysfunction: A Literature Review". Sexual Medicine Reviews 6 (1): 29–34. January 2018. doi:10.1016/j.sxmr.2017.07.002. PMID 28778697.
- ↑ "Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis". JAMA Psychiatry 70 (4): 436–443. April 2013. doi:10.1001/jamapsychiatry.2013.684. PMID 23446732.
- ↑ 50.0 50.1 "Early pregnancy exposure to selective serotonin reuptake inhibitors, risks of major structural malformations, and hypothesized teratogenic mechanisms". Expert Opinion on Drug Metabolism & Toxicology 11 (10): 1585–1597. 1 July 2015. doi:10.1517/17425255.2015.1063614. PMID 26135630.
- ↑ "Determination of citalopram enantiomers in human plasma by liquid chromatographic separation on a Chiral-AGP column". Journal of Chromatography. B, Biomedical Applications 685 (2): 299–305. October 1996. doi:10.1016/s0378-4347(96)00177-6. PMID 8953171.
- ↑ Baselt RC (2008). Disposition of toxic drugs and chemicals in man (8th ed.). Foster City, Ca: Biomedical Publications. pp. 552–553. ISBN 978-0962652370.
- ↑ "A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike?". International Clinical Psychopharmacology 29 (4): 185–196. July 2014. doi:10.1097/YIC.0000000000000023. PMID 24424469.
- ↑ "Escitalopram block of hERG potassium channels". Naunyn-Schmiedeberg's Archives of Pharmacology 387 (1): 23–32. January 2014. doi:10.1007/s00210-013-0911-y. PMID 24045971.
- ↑ "Serotonin and brain function: a tale of two receptors". Journal of Psychopharmacology 31 (9): 1091–1120. September 2017. doi:10.1177/0269881117725915. PMID 28858536.
- ↑ "How do antidepressants work? New perspectives for refining future treatment approaches" (in English). The Lancet. Psychiatry 4 (5): 409–418. May 2017. doi:10.1016/S2215-0366(17)30015-9. PMID 28153641.
- ↑ 57.0 57.1 Brunton L, Chabner B, Knollman B. Goodman and Gilman's The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.
- ↑ 58.0 58.1 "P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents". Neuropsychopharmacology 38 (11): 2209–2219. October 2013. doi:10.1038/npp.2013.120. PMID 23670590.
- ↑ "UpToDate". https://www.uptodate.com/content-not-available.
- ↑ "Escitalopram is a weak inhibitor of the CYP2D6-catalyzed O-demethylation of (+)-tramadol but does not reduce the hypoalgesic effect in experimental pain". Clinical Pharmacology and Therapeutics 86 (6): 626–633. December 2009. doi:10.1038/clpt.2009.154. PMID 19710642.
- ↑ 2006 Lippincott's Nursing Drug Guide. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo: Lippincott Williams & Wilkins. 2006. ISBN 978-1-58255-436-5.
- ↑ "The serotonin syndrome". The New England Journal of Medicine 352 (11): 1112–1120. March 2005. doi:10.1056/NEJMra041867. PMID 15784664.
- ↑ "The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects". British Journal of Clinical Pharmacology 60 (3): 287–290. September 2005. doi:10.1111/j.1365-2125.2005.02423.x. PMID 16120067.
- ↑ "Drug interactions between bupropion and Lexapro". https://www.drugs.com/drug-interactions/bupropion-with-lexapro-440-0-1013-565.html?professional=1.
- ↑ "Citalopram and escitalopram". Meyler's Side Effects of Drugs (6 ed.). Elsevier. 2016. p. 383.
- ↑ "2000 Annual Report. p 28 and 33". Lundbeck. 2000. http://www.materials.lundbeck.com/lundbeck/82/fullpdf/1.pdf.
- ↑ "FDA OKs Generic Depression Drug – Generic Version of Lexapro Gets Green Light". http://www.webmd.com/content/article/122/114778.htm.
- ↑ "US court upholds Lexapro patent". FirstWord. 14 July 2006. http://www.firstwordplus.com/Fws.do?articleid=7474B41ED0D14C20894E262219E24B62.
- ↑ "Forest Laboratories Receives Patent Term Extension for Lexapro" (Press release). PRNewswire-FirstCall. 2 March 2006. Archived from the original on 15 April 2009. Retrieved 19 January 2009.
- ↑ "Forest Laboratories: A Tale of Two Whistleblowers" article by Alison Frankel in The American Lawyer 27 February 2009
- ↑ United States of America v. Forest Laboratories Full text of the federal complaint filed in the US District Court for the district of Massachusetts
- ↑ "Drug Maker Is Accused of Fraud" article by Barry Meier and Benedict Carey in The New York Times 25 February 2009
- ↑ "Forest Laboratories, Inc. Provides Statement in Response to Complaint Filed by U.S. Government" Forest press-release. 26 February 2009.
- ↑ "Drug Maker Forest Pleads Guilty; To Pay More Than $313 Million to Resolve Criminal Charges and False Claims Act Allegations". 15 September 2010. https://www.justice.gov/opa/pr/drug-maker-forest-pleads-guilty-pay-more-313-million-resolve-criminal-charges-and-false.
- ↑ Gdziepolek.pl: Mozarin, zamienniki i podobne produkty Page accessed on 17 October 2020 (in Polish)
External links
- "A Peek at How Forest Laboratories Pushed Lexapro". 1 September 2009. https://www.nytimes.com/2009/09/02/business/02drug.html.
 |