Chemistry:Propofol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diprivan, others[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: very low (seizures) Psychological: no data |
| Addiction liability | Moderate[2] |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 95–99% |
| Metabolism | Liver glucuronidation |
| Onset of action | 15–30 seconds[4] |
| Elimination half-life | 1.5–31 hours[4] |
| Duration of action | ~5–10 minutes[4] |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
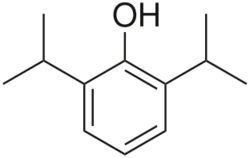
| Formula | C12H18O |
| Molar mass | 178.275 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | ΔGsolvH2O = -4.39kcal/mol[5] |
| |
| |
| (verify) | |
Propofol[6] is the active component of an intravenous anesthetic formulation used for induction and maintenance of general anesthesia. It is chemically termed 2,6-diisopropylphenol. The formulation was originally approved under the brand name Diprivan. Numerous generic offerings of this formulation now exist. Intravenous administration is used to induce unconsciousness after which anesthesia may be maintained using a combination of medications. It is manufactured as part of a sterile injectable emulsion formulation using soybean oil and lecithin, giving it a white milky coloration.[7]
Recovery from propofol-induced anesthesia is generally rapid and associated with less frequent side effects[8] (e.g. drowsiness, nausea, vomiting) compared to other anesthetic agents. Propofol may be used prior to diagnostic procedures requiring anesthesia, in the management of refractory status epilepticus, and for induction and/or maintenance of anesthesia prior to and during surgeries. It may be administered as a bolus or an infusion, or some combination of the two.
First synthesized in 1973, by John B. Glen, a British veterinary anesthesiologist working for Imperial Chemical Industries (ICI, now AstraZeneca).[9] In 1986 propofol was introduced for therapeutic use as a lipid emulsion in the United Kingdom and New Zealand. Propofol (Diprivan) received FDA approval in October 1989. It is on the World Health Organization's List of Essential Medicines.[10]
Uses
Anesthesia
Since its introduction, propofol has become almost exclusively the drug of choice of anesthesiologists for the induction and maintenance of general anesthesia, having largely replaced other agents.[11]
It is often administered as part of an anesthesia maintenance technique called total intravenous anesthesia, using either manually programmed infusion pumps or computer-controlled infusion pumps in a process called target controlled infusion (TCI).[12]
Propofol is also used to sedate individuals who are receiving mechanical ventilation but not undergoing surgery, such as patients in the intensive care unit.[13] In critically ill patients, propofol is superior to lorazepam both in effectiveness and overall cost.[14] Propofol is relatively inexpensive compared to medications of similar use due to shorter ICU stay length.[14] One of the reasons propofol is thought to be more effective (although it has a longer half-life than lorazepam) is that studies have found that benzodiazepines like midazolam and lorazepam tend to accumulate in critically ill patients, prolonging sedation.[14]
Propofol has also been suggested as a sleep aid in critically ill adults in an ICU setting; however, the effectiveness of this medicine in replicating the mental and physical aspects of sleep for people in the ICU is not clear.[13]
Propofol can be administered via a peripheral IV or central line. Propofol is often paired with fentanyl (for pain relief) in intubated and sedated people.[15] The two drugs are molecularly compatible in an IV mixture form.[15]
Propofol is also used for deepening of anesthesia in order to relieve laryngospasm. It may be used alone or followed by succinylcholine. Its use can avoid the need for paralysis and in some instances the potential side-effects of succinylcholine.[16]
Routine procedural sedation
Propofol is safe and effective for gastrointestinal endoscopy procedures (colonoscopies etc.). Its use in these settings results in a faster recovery compared to midazolam.[17] It can also be combined with opioids or benzodiazepines.[18][19][20] Because of its rapid induction and recovery time, propofol is also widely used for sedation of infants and children undergoing MRI procedures.[21] It is also often used in combination with ketamine with minimal side effects.[22]
COVID-19
In March 2021, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for Propofol‐Lipuro 1% to maintain sedation via continuous infusion in people older than sixteen with suspected or confirmed COVID-19 who require mechanical ventilation in an intensive care unit ICU setting.[23][24][25][26] During the public health emergency, it was considered infeasible to limit Fresenius Propoven 2% Emulsion or Propofol-Lipuro 1% to patients with suspected or confirmed COVID-19, so it was made available to all ICU patients under mechanical ventilation.[26] This EUA has since been revoked.[26]
Status epilepticus
Status epilepticus may be defined as seizure activity lasting beyond five minutes needing anticonvulsant medication. Several guidelines recommend the use of propofol for the treatment of refractory status epilepticus.[27]
Other uses
Assisted death in Canada
A lethal dose of propofol is used for medical assistance in dying in Canada to quickly induce deep coma and death, but rocuronium is always given as a paralytic ensuring death, even when the patient has died as a result of initial propofol overdose. [28]
Capital punishment
Use of this compound as part of an execution protocol have been considered though no individual has been executed using this agent. This is largely due to European manufactures and governments banning the exportation of this compound for such use.[29][30]
Recreational use
Recreational use of the drug via self-administration has been reported[31][32] but is relatively rare due to its potency and the level of monitoring required for safe use. Critically, a steep dose-response curve makes recreational use of propofol very dangerous, and deaths from self-administration continue to be reported.[33][34] The short-term effects sought via recreational use include mild euphoria, hallucinations, and disinhibition.[35][36]
Recreational use of the drug has been described among medical staff, such as anesthetists who have access to the drug.[37][38] It is reportedly more common among anesthetists on rotations with short rest periods, as usage generally produces a well-rested feeling.[39] Long-term use has been reported to result in addiction.[37][40]
Attention to the risks of off-label use of propofol increased in August 2009 due to the Los Angeles County coroner's conclusion that musician Michael Jackson died from a mixture of propofol and the benzodiazepine drugs lorazepam, midazolam, and diazepam on 25 June 2009.[41][42][43][44] According to a 22 July 2009 search warrant affidavit unsealed by the district court of Harris County, Texas, Jackson's physician, Conrad Murray, administered 25 milligrams of propofol diluted with lidocaine shortly before Jackson's death.[42][43][45]
Manufacturing
Propofol as a commercial sterile emulsified formulation is considered difficult to manufacture.[46][47][48]
It was initially formulated in Cremophor for human use, but this original formulation was implicated in an unacceptable number of anaphylactic events. It was eventually manufactured as a 1% emulsion in soybean oil.[49] Sterile emulsions represent complex formulation, the stability of which is dependent on the interplay of many factors such as micelle size and distribution.[50] [51][52]
Side effects
One of propofol's most common side effects is pain on injection, especially in smaller veins. This pain arises from activation of the pain receptor, TRPA1,[53] found on sensory nerves and can be mitigated by pretreatment with lidocaine.[54] Less pain is experienced when infused at a slower rate in a large vein (antecubital fossa). Patients show considerable variability in their response to propofol, at times showing profound sedation with small doses.
Additional side effects include low blood pressure related to vasodilation, transient apnea following induction doses, and cerebrovascular effects. Propofol has more pronounced hemodynamic effects relative to many intravenous anesthetic agents.[55] Reports of blood pressure drops of 30% or more are thought to be at least partially due to inhibition of sympathetic nerve activity.[56] This effect is related to the dose and rate of propofol administration. It may also be potentiated by opioid analgesics.[57]
Propofol can also cause decreased systemic vascular resistance, myocardial blood flow, and oxygen consumption, possibly through direct vasodilation.[58] There are also reports that it may cause green discoloration of the urine.[59]
Although propofol is widely used in the adult ICU setting, the side effects associated with medication seem to be more concerning in children. In the 1990s, multiple reported deaths of children in ICUs associated with propofol sedation prompted the FDA to issue a warning.[60]
As a respiratory depressant, propofol frequently produces apnea. The persistence of apnea can depend on factors such as premedication, dose administered, and rate of administration, and may sometimes persist for longer than 60 seconds.[61] Possibly as the result of depression of the central inspiratory drive, propofol may produce significant decreases in respiratory rate, minute volume, tidal volume, mean inspiratory flow rate, and functional residual capacity.[55]
Propofol administration also results in decreased cerebral blood flow, cerebral metabolic oxygen consumption, and intracranial pressure.[62] In addition, propofol may decrease intraocular pressure by as much as 50% in patients with normal intraocular pressure.[63]
A more serious but rare side effect is dystonia.[64] Mild myoclonic movements are common, as with other intravenous hypnotic agents. Propofol appears to be safe for use in porphyria, and has not been known to trigger malignant hyperpyrexia.[citation needed]
Propofol is also reported to induce priapism in some individuals,[65][66] and has been observed to suppress REM sleep stage and to worsen the poor sleep quality in some patients.[67]
Rare side effects include:[68]
- anxiety
- changes in vision
- cloudy urine
- coughing up blood
- delirium or hallucinations
- difficult urination
- difficulty swallowing
- dry eyes, mouth, nose, or throat
As with any other general anesthetic agent, propofol should be administered only where appropriately trained staff and facilities for monitoring are available, as well as proper airway management, a supply of supplemental oxygen, artificial ventilation, and cardiovascular resuscitation.[69]
Because of propofol's formulation (using lecithin and soybean oil) it is prone to bacterial contamination, despite the presence of the bacterial inhibitor benzyl alcohol, consequently - some hospital facilities require the IV tubing (of continuous propofol infusions) to be changed after 12 hours. This is a preventive measure against microbial growth and potential infection.[70]
Propofol infusion syndrome
A rare, but serious, side effect is propofol infusion syndrome. This potentially lethal metabolic derangement has been reported in critically ill patients after a prolonged infusion of high-dose propofol, sometimes in combination with catecholamines and/or corticosteroids.[71]
Interactions
The respiratory effects of propofol are increased if given with other respiratory depressants, including benzodiazepines.[72]
Pharmacology
Pharmacodynamics
Propofol has been proposed to have several mechanisms of action,[73][74][75] both through potentiation of GABAA receptor activity and therefore acting as a GABAA receptor positive allosteric modulator, thereby slowing the channel-closing time. At high doses, propofol may be able to activate GABAA receptors in the absence of GABA, behaving as a GABAA receptor agonist as well.[76][77][78] Propofol analogs have been shown to also act as sodium channel blockers.[79][80] Some research has also suggested that the endocannabinoid system may contribute significantly to propofol's anesthetic action and to its unique properties, as endocannabinoids also play an important role in the physiologic control of sleep, pain processing and emesis.[81][82] An EEG study on patients undergoing general anesthesia with propofol found that it causes a prominent reduction in the brain's information integration capacity.[83]
Propofol is an inhibitor of the enzyme fatty acid amide hydrolase, which metabolizes the endocannabinoid anandamide (AEA). Activation of the endocannabinoid system by propofol, possibly via inhibition of AEA catabolism, generates a significant increase in the whole-brain content of AEA, contributing to the sedative properties of propofol via CB1 receptor activation.[84] This may explain the psychotomimetic and antiemetic properties of propofol. By contrast, there is a high incidence of postoperative nausea and vomiting after administration of volatile anesthetics, which contribute to a significant decrease in the whole-brain content of AEA that can last up to forty minutes after induction.[82]
Pharmacokinetics
Propofol is highly protein-bound in vivo and is metabolized by conjugation in the liver.[85] The half-life of elimination of propofol has been estimated to be between 2 and 24 hours. However, its duration of clinical effect is much shorter, because propofol is rapidly distributed into peripheral tissues. When used for IV sedation, a single dose of propofol typically wears off within minutes. Onset is rapid, in as little as 15–30 seconds.[4] Propofol is versatile; the drug can be given for short or prolonged sedation, as well as for general anesthesia. Its use is not associated with nausea as is often seen with opioid medications. These characteristics of rapid onset and recovery along with its amnestic effects[86] have led to its widespread use for sedation and anesthesia.
History
John B. Glen, a veterinarian and researcher at Imperial Chemical Industries (ICI), spent thirteen years developing propofol, an effort for which he was awarded the 2018 Lasker Award for clinical research.
Originally developed as ICI 35868, propofol was chosen after extensive evaluation and structure–activity relationship studies of the anesthetic potencies and pharmacokinetic profiles of a series of ortho-alkylated phenols.[87]
First identified as a drug candidate in 1973, propofol entered clinical trials in 1977, using a form solubilized in cremophor EL.[88] However, due to anaphylactic reactions to cremophor, this formulation was withdrawn from the market and subsequently reformulated as an emulsion of a soya oil and propofol mixture in water. The emulsified formulation was relaunched in 1986 by ICI (whose pharmaceutical division later became a constituent of AstraZeneca) under the brand name Diprivan. The preparation contains 1% propofol, 10% soybean oil, and 1.2% purified egg phospholipid as an emulsifier, with 2.25% glycerol as a tonicity-adjusting agent, and sodium hydroxide to adjust the pH. Diprivan contains EDTA, a common chelation agent, that also acts alone (bacteriostatically against some bacteria) and synergistically with some other antimicrobial agents. Newer generic formulations contain sodium metabisulfite as an antioxidant and benzyl alcohol as antimicrobial agent. Propofol emulsion is an opaque white fluid due to the scattering of light from the emulsified micelle formulation.
Developments
A water-soluble prodrug form, fospropofol, has been developed and tested with positive results. Fospropofol is rapidly broken down by the enzyme alkaline phosphatase to form propofol. Marketed as Lusedra, this formulation may not produce the pain at injection site that often occurs with the conventional form of the drug. The U.S. Food and Drug Administration (FDA) approved the product in 2008.[89]
By incorporation of an azobenzene unit, a photoswitchable version of propofol (AP2) was developed in 2012 that allows for optical control of GABAA receptors with light.[90] In 2013, a propofol binding site on mammalian GABAA receptors has been identified by photolabeling using a diazirine derivative.[91] Additionally, it was shown that the hyaluronan polymer present in the synovia can be protected from free-radical depolymerization by propofol.[92]
Ciprofol is another derivative of propofol that is 4–6 times more potent than propofol. Currently undergoing Phase III trials, ciprofol appears to have a lower incidence of injection site pain and respiratory depression than propofol.[93]
Propofol has also been studied for treatment resistant depression.[94]
References
- ↑ "Propofol". https://www.drugs.com/international/propofol.html.
- ↑ "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse 40 (6): 428–437. November 2014. doi:10.3109/00952990.2014.933840. PMID 25083822. "Propofol is a general anesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both ΔFosB induction and addiction (ethanol and nicotine), similar ΔFosB expression was apparent when propofol was given to rats. Moreover, this cascade was shown to act via the dopamine D1 receptor in the NAc, suggesting that propofol has abuse potential (119)".
- ↑ "Diprivan- propofol injection, emulsion". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5cec3b97-5183-4a9d-accd-21e76c99d3dd.
- ↑ 4.0 4.1 4.2 4.3 "Propofol". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/Propofol.html.
- ↑ "Atomistic models of general anesthetics for use in in silico biological studies". The Journal of Physical Chemistry B (American Chemical Society (ACS)) 118 (42): 12075–12086. October 2014. doi:10.1021/jp502716m. PMID 25303275.
- ↑ "Propofol" (in en). PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/4943.
- ↑ "Making Stable, Sterile Propofol" (in en-us). https://www.microfluidics-mpt.com/blog/making-stable-sterile-propofol.
- ↑ "Propofol" (in en). https://go.drugbank.com/drugs/DB00818.
- ↑ "Try, try, and try again: personal reflections on the development of propofol". British Journal of Anaesthesia 123 (1): 3–9. July 2019. doi:10.1016/j.bja.2019.02.031. PMID 30982566.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Propofol - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/propofol.
- ↑ "Propofol anaesthesia via target controlled infusion or manually controlled infusion: effects on the bispectral index as a measure of anaesthetic depth". Anaesthesia and Intensive Care 29 (6): 579–584. December 2001. doi:10.1177/0310057X0102900602. ISSN 0310-057X. PMID 11771598.
- ↑ 13.0 13.1 "Propofol for the promotion of sleep in adults in the intensive care unit". The Cochrane Database of Systematic Reviews 1 (1): CD012454. January 2018. doi:10.1002/14651858.CD012454.pub2. PMID 29308828.
- ↑ 14.0 14.1 14.2 "Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation". Critical Care Medicine 36 (3): 706–714. March 2008. doi:10.1097/CCM.0B013E3181544248. PMID 18176312.
- ↑ 15.0 15.1 "Compatibility of propofol, fentanyl, and vecuronium mixtures designed for potential use in anesthesia and patient transport". Journal of Clinical Anesthesia 8 (4): 329–336. June 1996. doi:10.1016/0952-8180(96)00043-8. PMID 8695138.
- ↑ "Laryngospasm in anaesthesia". Continuing Education in Anaesthesia Critical Care & Pain 14 (2): 47–51. April 2014. doi:10.1093/bjaceaccp/mkt031.
- ↑ "A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures". Gastrointestinal Endoscopy 67 (6): 910–923. May 2008. doi:10.1016/j.gie.2007.12.046. PMID 18440381.
- ↑ Canadian National Formulary 2010
- ↑ Appleton & Lange's 1999 drug guide. Stamford, CT: Appleton & Lange. 1999. ISBN 978-0-8385-0371-3.
- ↑ Numorphan® (oxymorphone) package insert (English), Endo 2009
- ↑ "Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging". British Journal of Anaesthesia 101 (2): 239–243. August 2008. doi:10.1093/bja/aen153. PMID 18534971.
- ↑ "Ketamine-Propofol Versus Propofol Alone for Procedural Sedation in the Emergency Department: A Systematic Review and Meta-analysis". Academic Emergency Medicine 22 (9): 1003–1013. September 2015. doi:10.1111/acem.12737. PMID 26292077.
- ↑ "Propofol-Lipuro 1% (propofol) Injectable emulsion for infusion – 1,000 mg in 100 ml (10 mg /ml) : Fact Sheet for health Care Providers". https://www.bbraunusa.com/content/dam/b-braun/us/website/company/covid-files/210319_Propofol_EUA_Submission_to_FDA_hcp.pdf.
- ↑ "Letter RE: Emergency Use Authorization 096". https://www.fda.gov/media/146680/download.
- ↑ "Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Propofol-Lipuro 1% Injectable Emulsion for Infusion". https://www.fda.gov/media/146681/download.
- ↑ 26.0 26.1 26.2 "Emergency Use Authorization". https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
- ↑ "Seizures and epilepsy.". Harrison's Principles of Internal Medicine (21st ed.). McGraw Hill. 2022. ISBN 978-1-264-26851-1. https://accessmedicine.mhmedical.com/content.aspx?bookid=3095§ionid=265447874.
- ↑ "Medical Assistance in Dying (MAiD): Protocols and Procedures Handbook". Divisions of Family Practice. Comox Valley, British Columbia. May 2017. https://divisionsbc.ca/sites/default/files/51936/Medical%20Assistance%20in%20Dying%20(MAID)%20Protocols%20and%20Procedures%20Handbook%20Comox%20Valley%202017%20-%202nd%20edition_0.pdf.
- ↑ "The role of anaesthesiologists in lethal injection: a call to action". Lancet 395 (10225): 749–754. February 2020. doi:10.1016/S0140-6736(19)32986-1. PMID 32014115.
- ↑ "Lethal injection: Secretive US states resort to untested drugs" (in en-GB). BBC News. 2013-11-15. https://www.bbc.com/news/world-us-canada-24935868.
- ↑ "Brugada-like EKG pattern and myocardial effects in a chronic propofol abuser". Clinical Toxicology 47 (4): 358–363. April 2009. doi:10.1080/15563650902887842. PMID 19514884.
- ↑ "With High-Profile Death, Focus on High-Risk Drug". The New York Times. 6 August 2009. https://www.nytimes.com/2009/08/07/us/07propofol.html.
- ↑ "Death after excessive propofol abuse". International Journal of Legal Medicine 114 (4–5): 248–251. 2001. doi:10.1007/s004149900129. PMID 11355404.
- ↑ "Lethal self administration of propofol (Diprivan). A case report and review of the literature". Forensic Science International 167 (1): 56–58. March 2007. doi:10.1016/j.forsciint.2005.12.027. PMID 16431058.
- ↑ Martindale: The Complete Drug Reference (34th ed.). London: Pharmaceutical Press. 2005. pp. 1305–1307. ISBN 978-0-85369-550-9.
- ↑ "General anesthetics and anesthetic gases.". Meyler's Side Effects of Drugs (14th ed.). Amsterdam: Elsevier Science. 2000. p. 330. ISBN 978-0-444-50093-9.
- ↑ 37.0 37.1 "Pharmacological and clinical evidences on the potential for abuse and dependence of propofol: a review of the literature". Fundamental & Clinical Pharmacology 21 (5): 459–466. October 2007. doi:10.1111/j.1472-8206.2007.00497.x. PMID 17868199.
- ↑ "Propofol: dancing with a "White Rabbit."". California Society Anesthesiology Bulletin 57 (Spring): 61–63. 2008. https://csahq.org/docs/default-source/news-and-events-docs/csa-bulletin-docs/spring-2008/propofol_57_2.pdf. Retrieved 24 November 2014.
- ↑ "Concerns mount over misuse of anaesthetic propofol among US health professionals". BMJ 339: b3673. September 2009. doi:10.1136/bmj.b3673. PMID 19737827.
- ↑ "A case report of propofol dependence in a physician". Journal of Psychoactive Drugs 40 (2): 215–217. June 2008. doi:10.1080/02791072.2008.10400634. PMID 18720673.
- ↑ "Jackson's Death Ruled a Homicide". The New York Times. 28 August 2009. https://www.nytimes.com/2009/08/29/us/29jackson.html.
- ↑ 42.0 42.1 "Coroner Attributes Michael Jackson's Death to Propofol". The Washington Post. 25 August 2009. https://www.washingtonpost.com/wp-dyn/content/article/2009/08/24/AR2009082402193.html.
- ↑ 43.0 43.1 "Coroner's Findings in Jackson Death Revealed". The New York Times. 24 August 2009. http://artsbeat.blogs.nytimes.com/2009/08/24/coroners-findings-in-jackson-death-revealed/?hp.
- ↑ "Jackson's Death: How Dangerous Is Propofol?". Time. 25 August 2009. http://www.time.com/time/arts/article/0,8599,1918363,00.html. Retrieved 22 May 2010.
- ↑ "Michael Jackson search warrant". Scribd. https://www.scribd.com/doc/19058649/Michael-Jackson-search-warrant.
- ↑ "Development of a compounded propofol nanoemulsion using multiple non-invasive process analytical technologies". International Journal of Pharmaceutics 640: 122960. June 2023. doi:10.1016/j.ijpharm.2023.122960. PMID 37061210.
- ↑ "Infectious Disease Risk Associated with Contaminated Propofol Anesthesia, 1989-2014(1)" (in en-us). Emerging Infectious Diseases 22 (6): 981–992. June 2016. doi:10.3201/eid2206.150376. PMID 27192163.
- ↑ Pramanick S, Gurjar S, Mehta SS, "Pharmaceutical composition of propofol", WO patent 2014033751A2, issued 2014-03-06, assigned to Emcure Pharmaceuticals Limited
- ↑ "Try, try, and try again: personal reflections on the development of propofol". British Journal of Anaesthesia 123 (1): 3–9. July 2019. doi:10.1016/j.bja.2019.02.031. PMID 30982566.
- ↑ "Hospira recalls lot of Propofol Injectable Emulsion" (in en). https://www.europeanpharmaceuticalreview.com/news/173149/hospira-recalls-lot-of-propofol-injectable-emulsion/.
- ↑ "Understanding Emulsion Formulation | Ascendia Pharmaceuticals" (in en). https://ascendiapharma.com/newsroom/2021/11/08/emulsion-formulation.
- ↑ "Stability of Emulsion - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/chemical-engineering/stability-of-emulsion#:~:text=The%20factors%20that%20influence%20the,increasing%20the%20stability%20of%20emulsion..
- ↑ "General anesthetics activate a nociceptive ion channel to enhance pain and inflammation". Proceedings of the National Academy of Sciences of the United States of America 105 (25): 8784–8789. June 2008. doi:10.1073/pnas.0711038105. PMID 18574153.
- ↑ "Propofol Drug Information, Professional". m drugs.com. https://www.drugs.com/MMX/Propofol.html.
- ↑ 55.0 55.1 "Propofol: a new intravenous anesthetic". Anesthesiology 71 (2): 260–277. August 1989. doi:10.1097/00000542-198908000-00015. PMID 2667401.
- ↑ "Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation?". Anesthesiology 86 (1): 64–72. January 1997. doi:10.1097/00000542-199701000-00010. PMID 9009941.
- ↑ "New awakening in anaesthesia—at a price". Lancet 329 (8548): 1469–70. 1987. doi:10.1016/s0140-6736(87)92214-8.
- ↑ "Clinical pharmacology of propofol: an intravenous anesthetic agent". DICP 23 (10): 743–749. October 1989. doi:10.1177/106002808902301001. PMID 2683416.
- ↑ "Green discoloration of urine after propofol infusion". Korean Journal of Anesthesiology 65 (2): 177–179. August 2013. doi:10.4097/kjae.2013.65.2.177. PMID 24024005.
- ↑ "Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports". BMJ 305 (6854): 613–616. September 1992. doi:10.1136/bmj.305.6854.613. PMID 1393073.
- ↑ "Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic". Drugs 35 (4): 334–372. April 1988. doi:10.2165/00003495-198835040-00002. PMID 3292208.
- ↑ "Pharmacokinetics of propofol in adult patients undergoing coronary revascularization. The Multicenter Study of Perioperative Ischemia Research Group". Anesthesiology 84 (6): 1288–1297. June 1996. doi:10.1097/00000542-199606000-00003. PMID 8669668.
- ↑ "New intravenous anaesthetics and neuromuscular blocking drugs. A review of their properties and clinical use". Drugs 34 (1): 98–135. July 1987. doi:10.2165/00003495-198734010-00004. PMID 3308413.
- ↑ "Dystonic reaction to propofol attenuated by benztropine (cogentin)". Anesthesia and Analgesia 94 (5): 1237–40, table of contents. May 2002. doi:10.1097/00000539-200205000-00034. PMID 11973196.
- ↑ "Propofol-induced priapism, a case confirmed with rechallenge". The Annals of Pharmacotherapy 40 (5): 980–982. May 2006. doi:10.1345/aph.1G555. PMID 16638914.
- ↑ "Successful treatment of propofol-induced priapism with distal glans to corporal cavernosal shunt". Urology 74 (1): 113–115. July 2009. doi:10.1016/j.urology.2008.12.066. PMID 19371930.
- ↑ "Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study". Intensive Care Medicine 38 (10): 1640–1646. October 2012. doi:10.1007/s00134-012-2623-z. PMID 22752356.
- ↑ "Propofol (Intravenous Route) Side Effects - Mayo Clinic". https://www.mayoclinic.org/drugs-supplements/propofol-intravenous-route/side-effects/drg-20488192?p=1.
- ↑ "AstraZeneca – United States Home Page". .astrazeneca-us.com. http://www1.astrazeneca-us.com/pi/diprivan.pdf.
- ↑ "Healthcare students interprofessional critical event/disaster response course". American Journal of Disaster Medicine 12 (1): 11–26. 1 January 2017. doi:10.5055/ajdm.2017.0254. PMID 28822211.
- ↑ "The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome". Intensive Care Medicine 29 (9): 1417–1425. September 2003. doi:10.1007/s00134-003-1905-x. PMID 12904852.
- ↑ "Propofol Linked to Michael Jackson's Death". WebMD. 24 August 2009. http://www.webmd.com/pain-management/news/20090824/propofol-linked-to-michael-jacksons-death.
- ↑ "Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery". Current Medicinal Chemistry 7 (2): 249–271. February 2000. doi:10.2174/0929867003375335. PMID 10637364.
- ↑ "The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties". CNS Neuroscience & Therapeutics 14 (2): 95–106. Summer 2008. doi:10.1111/j.1527-3458.2008.00043.x. PMID 18482023.
- ↑ "Propofol". Modern Anesthetics. Handbook of Experimental Pharmacology. 182. 2008. pp. 227–52. doi:10.1007/978-3-540-74806-9_11. ISBN 978-3-540-72813-9.
- ↑ "Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors". Journal of Medicinal Chemistry 41 (11): 1846–1854. May 1998. doi:10.1021/jm970681h. PMID 9599235.
- ↑ "General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility". The Journal of Pharmacology and Experimental Therapeutics 297 (1): 338–351. April 2001. PMID 11259561.
- ↑ "4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABA(A) receptor". Journal of Medicinal Chemistry 45 (15): 3210–3221. July 2002. doi:10.1021/jm010461a. PMID 12109905.
- ↑ "High-affinity block of voltage-operated rat IIA neuronal sodium channels by 2,6 di-tert-butylphenol, a propofol analogue". European Journal of Anaesthesiology 20 (3): 220–224. March 2003. doi:10.1017/s0265021503000371. PMID 12650493.
- ↑ "High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues". British Journal of Pharmacology 155 (2): 265–275. September 2008. doi:10.1038/bjp.2008.255. PMID 18574460.
- ↑ "Possible involvement of the endocannabinoid system in the actions of three clinically used drugs". Trends in Pharmacological Sciences 25 (2): 59–61. February 2004. doi:10.1016/j.tips.2003.12.001. PMID 15106622.
- ↑ 82.0 82.1 "Effects of General Anesthesia on Anandamide Blood Levels in Humans". Anesthesiology 104 (2): 273–277. 2006. doi:10.1097/00000542-200602000-00012. PMID 16436846. https://pubs.asahq.org/anesthesiology/article/104/2/273/630/Effects-of-General-Anesthesia-on-Anandamide-Blood. Retrieved 2022-12-11.
- ↑ "Propofol induction reduces the capacity for neural information integration: implications for the mechanism of consciousness and general anesthesia". Consciousness and Cognition 18 (1): 56–64. March 2009. doi:10.1016/j.concog.2008.10.005. PMID 19054696.
- ↑ "The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase". British Journal of Pharmacology 139 (5): 1005–1013. July 2003. doi:10.1038/sj.bjp.0705334. PMID 12839875.
- ↑ "Propofol metabolites in man following propofol induction and maintenance". British Journal of Anaesthesia 88 (5): 653–658. May 2002. doi:10.1093/bja/88.5.653. PMID 12067002.
- ↑ "The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations". Anesthesiology 87 (4): 749–764. October 1997. doi:10.1097/00000542-199710000-00007. PMID 9357875.
- ↑ "Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents". Journal of Medicinal Chemistry 23 (12): 1350–1357. December 1980. doi:10.1021/jm00186a013. PMID 7452689.
- ↑ Lasker Foundation. "Discovery and development of propofol, a widely used anesthetic". http://www.laskerfoundation.org/awards/show/discovery-and-development-propofol-widely-used-anesthetic/.
- ↑ "Drugs@FDA: FDA Approved Drug Products". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
- ↑ "Azo-propofols: photochromic potentiators of GABA(A) receptors". Angewandte Chemie 51 (42): 10500–10504. October 2012. doi:10.1002/anie.201205475. PMID 22968919.
- ↑ "A propofol binding site on mammalian GABAA receptors identified by photolabeling". Nature Chemical Biology 9 (11): 715–720. November 2013. doi:10.1038/nchembio.1340. PMID 24056400.
- ↑ "Hyaluronan can be protected from free-radical depolymerisation by 2,6-diisopropylphenol, a novel radical scavenger". Biochemical and Biophysical Research Communications 193 (3): 927–933. June 1993. doi:10.1006/bbrc.1993.1714. PMID 8391811.
- ↑ "The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study". BMC Anesthesiology 22 (1): 245. August 2022. doi:10.1186/s12871-022-01782-7. PMID 35922771.
- ↑ "A synopsis of multitarget therapeutic effects of anesthetics on depression". European Journal of Pharmacology 957: 176032. October 2023. doi:10.1016/j.ejphar.2023.176032. PMID 37660970.
External links
- "Propofol". U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/propofol.
- & Roger James"Pharmaceutical Compositions" GB patent 1472793, published 1977-05-04, assigned to Imperial Chemical Industries Ltd
 |