Chemistry:Ibotenic acid: Difference between revisions
(add) |
(No difference)
|
Latest revision as of 01:22, 16 March 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
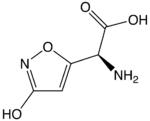
(S)-2-Amino-2-(3-hydroxyisoxazol-5-yl)acetic acid
| |
| Other names
Ibotenic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6N2O4 | |
| Molar mass | 158.113 g·mol−1 |
| Melting point | 151-152 °C (anhydrous) 144-146 °C (monohydrate) |
| H2O: 1 mg/mL 0.1 M NaOH: 10.7 mg/mL 0.1 M HCl: 4.7 mg/mL | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H301 | |
| P264, P270, P301+316Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ibotenic acid or (S)-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a chemical compound and psychoactive drug which occurs naturally in Amanita muscaria and related species of mushrooms typically found in the temperate and boreal regions of the northern hemisphere. It is a prodrug of muscimol, broken down by the liver to that much more stable compound.[2] It is a conformationally-restricted analogue of the neurotransmitter glutamate, and due to its structural similarity to this neurotransmitter, acts as a non-selective glutamate receptor agonist.[3] Because of this, ibotenic acid can be a powerful neurotoxin in high doses, and is employed as a "brain-lesioning agent" through cranial injections in scientific research.[4][5] The neurotoxic effects appear to be dose-related and risks are unclear through consumption of ibotenic-acid containing fungi, although thought to be negligible in small doses.[6][better source needed]
Pharmacology
Ibotenic acid acts as a potent agonist of the NMDA and group I (mGluR1 and mGluR5) and II (mGluR2 and mGluR3) metabotropic glutamate receptors.[3][7] It is inactive at group III mGluRs.[8] Ibotenic acid also acts as a weak agonist of the AMPA and kainate receptors.[3][7] In addition, due to in vivo decarboxylation into muscimol, it acts indirectly as a potent GABAA and GABAA-ρ receptor agonist.[7] Unlike muscimol—the principal psychoactive constituent of Amanita muscaria that is understood to cause sedation and delirium—ibotenic acid's psychoactive effects are not known independent of its serving as a prodrug to muscimol, however it may be speculated that it would act as a stimulant.[9][10]
Biological properties
Mechanism of action
Ibotenic acid is an agonist of glutamate receptors, specifically at both the N-methyl-D-aspartate, or NMDA, and trans-ACPD receptor sites in multiple systems in the central nervous system. Ibotenic neurotoxicity can be enhanced by glycine and blocked by dizocilpine. Dizocilpine acts as an uncompetitive antagonist at NMDA receptors.[11]
Ibotenic acid toxicity comes from activation of the NMDA receptors. NMDA receptors are related to synaptic plasticity and work with metabotropic glutamate receptors to establish long term potentiation or LTP. The process of long term potentiation is believed to be related to the acquisition of information. The NMDA receptor functions properly by allowing Ca2+ ions to pass through after activation at the receptor site.
The binding of ibotenic acid allows excess Ca2+ into the system which results in neuronal cell death. Ca2+ also activates CaM-KII or Ca2+/Calmodulin Kinase which phosphorylates multiple enzymes. The activated enzymes then begin producing reactive oxygen species which damages surrounding tissue. The excess Ca2+ results in the enhancement of the mitochondrial electron transport system which will further increase the number of reactive oxygen species.[12]
Biological effects
Ibotenic acid typically affects both NMDA and APCD or metabolotropic quisqualate receptor sites in the central nervous system.[11] Due to their targeting of these systems the symptoms associated with Ibotenic acid poisoning are often related to perception and control.
At least some ingested ibotenic acid is likely decarboxylated into muscimol so some of the effects of ingesting ibotenic acid are similar to muscimol's effects.[13] Symptoms associated with ibotenic acid are usually onset within 30–60 minutes and include a range of nervous system effects. The most common symptoms include nausea, vomiting, and drowsiness. However, after the first hour symptoms begin to include confusion, euphoria, visual and auditory distortions, sensations of floating, and retrograde amnesia.[14]
Symptoms are slightly different for children, typically beginning after 30–180 minutes. Dominant symptoms in children include ataxia, obtundation, and lethargy. Seizures are occasionally reported, however, more commonly with children.[14]
Treatment
Treatment of ibotenic acid toxicity centres around supportive care and treatment of symptoms; no antidote is available. Gastric decontamination with activated charcoal or gastric lavage can be of benefit if the patient presents early. The psychotropic effects and hallucinations ibotenic acid and its metabolite muscimol produce are best managed in a quiet environment with minimal stimulation. Benzodiazepines can be of benefit in agitated or panicked patients; they can also be used to control seizures if they occur. (Benzodiazepines as a GABA-A PAM interacts with Muscimol as a GABA-A agonist and may cause a significantly increased risk of depressant effects.) Airway management may be required if sedation is profound. Symptoms usually resolve within a few hours of ingestion but can last for days following significant exposures.[15]
Monitoring for the presence of brain lesions may be required following a large or repeated exposure. Other measures may be required if the patient has been exposed to a mushroom such as Amanita muscaria as other active compounds may be present.[16]
Use in research
Ibotenic acid used for the lesioning of rat's brains is kept frozen in a phosphate-buffered Saline Solution at a pH of 7.4, and can be kept for up to a year with no loss in toxicity. Injection of .05-.1 microliters of Ibotenic acid into the hippocampus at a rate of .1 microliter/min resulted in semi-selective lesioning. Hippocampal lesioning led to a considerable loss of cells in pyramidal cells (CA1-CA3) as well as granule cells in the dentate gyrus. Ibotenic acid lesioning also causes some damage to axons along the perforant pathway.
Typically, when lesioning is done with other chemicals the subject cannot relearn a task. However, due to Ibotenic acid's reactivity with glutamate receptors such as the NMDA receptor, Ibotenic acid lesioning does allow the subject to relearn tasks. Ibotenic acid lesioning is thus preferred in studies where re-learning a task after lesioning is essential. Compared to other lesioning agents, Ibotenic acid is one of the most site-specific; however, less-damaging alternatives are presently sought.[17]
Biosynthesis
Ibotenic acid's biosynthetic genes are organized in a physically linked biosynthetic gene cluster. The biosynthetic pathway is initiated by hydroxylation of glutamic acid by a dedicated Fe(II)/2-oxoglutarate-dependent oxygenase. The reaction yields threo-3-hydroxyglutamic acid, which is converted into ibotenic acid, likely by enzymes encoded in the biosynthetic gene cluster.[18]
See also
References
- ↑ "Ibotenic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/1233#section=Safety-and-Hazards.
- ↑ Stebelska, Katarzyna (August 2013). "Fungal Hallucinogens Psilocin, Ibotenic Acid, and Muscimol: Analytical Methods and Biologic Activities" (in en-US). Therapeutic Drug Monitoring 35 (4): 420–442. doi:10.1097/FTD.0b013e31828741a5. ISSN 0163-4356. PMID 23851905. https://journals.lww.com/drug-monitoring/Abstract/2013/08000/Fungal_Hallucinogens_Psilocin,_Ibotenic_Acid,_and.2.aspx.
- ↑ 3.0 3.1 3.2 Tommy Liljefors; Povl Krogsgaard-Larsen; Ulf Madsen (25 July 2002). Textbook of Drug Design and Discovery, Third Edition. CRC Press. pp. 263–. ISBN 978-0-415-28288-8. https://books.google.com/books?id=EL-UI6t8omQC&pg=PA263.
- ↑ Becker, A; Grecksch, G; Bernstein, HG; Höllt, V; Bogerts, B (1999). "Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis". Psychopharmacology 144 (4): 333–8. doi:10.1007/s002130051015. PMID 10435405.
- ↑ Isacson, O; Brundin, P; Kelly, PA; Gage, FH; Björklund, A (1984). "Functional neuronal replacement by grafted striatal neurones in the ibotenic acid-lesioned rat striatum". Nature 311 (5985): 458–60. doi:10.1038/311458a0. PMID 6482962. Bibcode: 1984Natur.311..458I.
- ↑ Filer, Crist N. (1 December 2018). "Ibotenic acid: on the mechanism of its conversion to [3H muscimol"] (in en). Journal of Radioanalytical and Nuclear Chemistry 318 (3): 2033–2038. doi:10.1007/s10967-018-6203-8. ISSN 1588-2780. https://doi.org/10.1007/s10967-018-6203-8.
- ↑ 7.0 7.1 7.2 Wantanabe (23 July 1999). Pharmacological Research on Traditional Herbal Medicines. CRC Press. pp. 107–. ISBN 978-90-5702-054-4. https://books.google.com/books?id=UExUOKw3EwYC&pg=PA107.
- ↑ "Ibotenic acid and thioibotenic acid: a remarkable difference in activity at group III metabotropic glutamate receptors". Eur. J. Pharmacol. 486 (3): 241–50. 2004. doi:10.1016/j.ejphar.2003.12.033. PMID 14985045.
- ↑ Chilton 1975; Theobald et al. 1968
- ↑ Chilton 1975; Ott 1976a
- ↑ 11.0 11.1 Zinkand, William; Moore, W.; Thompson, Carolann; Salama, Andre; Patel, Jitendra (February 1992). "Ibotenic acid mediates neurotoxicity and phosphoinositide hydrolysis by independent receptor mechanisms". Molecular and Chemical Neuropathology 16 (1–2): 1–10. doi:10.1007/bf03159956. PMID 1325800.
- ↑ Sureda, F.. "Excitotoxicity and the NMDA receptor". http://www.eurosiva.org/archive/vienna/abstracts/speakers/sureda.htm.
- ↑ Michelot, Didier; Melendez-Howell, Leda Maria (2003). "Amanita muscaria: chemistry, biology, toxicology, and ethnomycology". Mycological Research 107 (2): 131–146. doi:10.1017/s0953756203007305. PMID 12747324. http://www.davidmoore.org.uk/21st_Century_Guidebook_to_Fungi_PLATINUM/REPRINT_collection/Michelot_etal_A.muscaria_chemistry_biology_toxicology.pdf.
- ↑ 14.0 14.1 Duffy, Thomas. "Symptoms of ibotenic/muscimol poisoning (isoxazol poisoning)". MykoWeb. http://www.mykoweb.com/TFWNA/P-28.html.
- ↑ Rolston-Cregler, Louis. "Hallucinogenic Mushroom Toxicity". http://emedicine.medscape.com/article/817848-overview.
- ↑ Michelot D, Melendez-Howell LM (2003). "Amanita muscaria: chemistry, biology, toxicology, and ethnomycology.". Mycol Res 107 (Pt 2): 131-46. doi:10.1017/s0953756203007305. PMID 12747324.
- ↑ Jarrard, Leonard (2 February 1989). "On the use of ibotenic acid to lesion selectively different components of the hippocampal formation". Journal of Neuroscience Methods 29 (3): 251–259. doi:10.1016/0165-0270(89)90149-0. PMID 2477650.
- ↑ Obermaier, Sebastian; Müller, Michael (31 March 2020). "Ibotenic Acid Biosynthesis in the Fly Agaric Is Initiated by Glutamate Hydroxylation" (in en). Angewandte Chemie International Edition 59 (30): 12432–12435. doi:10.1002/anie.202001870. PMID 32233056.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |



