Revision as of 01:55, 23 June 2022 by imported>Corlink
(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
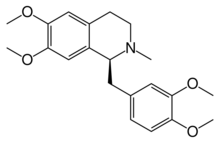
Laudanosine
|
|
| Names
|
Preferred IUPAC name
(1S)-1-[(3,4-Dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinoline |
| Other names
N-Methyl-1,2,3,4-tetrahydropapaverine
|
| Identifiers
|
|
|
|
|
|
|
| ChemSpider
|
|
| EC Number
|
|
|
|
|
| UNII
|
|
InChI=1S/C21H27NO4/c1-22-9-8-15-12-20(25-4)21(26-5)13-16(15)17(22)10-14-6-7-18(23-2)19(11-14)24-3/h6-7,11-13,17H,8-10H2,1-5H3/t17-/m0/s1  N NKey: KGPAYJZAMGEDIQ-KRWDZBQOSA-N  N NInChI=1/C21H27NO4/c1-22-9-8-15-12-20(25-4)21(26-5)13-16(15)17(22)10-14-6-7-18(23-2)19(11-14)24-3/h6-7,11-13,17H,8-10H2,1-5H3/t17-/m0/s1 Key: KGPAYJZAMGEDIQ-KRWDZBQOBO
|
CN1CCc2cc(c(cc2[C@@H]1Cc3ccc(c(c3)OC)OC)OC)OC
|
| Properties
|
|
|
C21H27NO4
|
| Molar mass
|
357.450 g·mol−1
|
| Melting point
|
89 °C (192 °F; 362 K)
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
| Infobox references
|
|
|
|
Tracking categories (test):
Laudanosine or N-methyltetrahydropapaverine is a recognized metabolite[1] of atracurium and cisatracurium. Laudanosine decreases the seizure threshold, and thus it can induce seizures if present at sufficient threshold concentrations; however such concentrations are unlikely to be produced consequent to chemodegradable metabolism of clinically administered doses of cisatracurium or atracurium.

Capsule of
Papaver somniferum showing latex (opium) exuding from incision. Laudanosine occurs naturally in small amounts (0.1%) in opium.
Laudanosine also occurs naturally in minute amounts (0.1%) in opium, from which it was first isolated in 1871.[2] Partial dehydrogenation of laudanosine will lead to papaverine, the alkaloid found in the opium poppy plant (Papaver somniferum).
Laudanosine is a benzyltetrahydroisoquinoline alkaloid. It has been shown to interact with GABA receptors, glycine receptors, opioid receptors, and nicotinic acetylcholine receptors,[1][3][4] but not benzodiazepine or muscarinic receptors, which are also involved in epilepsy and other types of seizures.[5]
References
|
|---|
{{Navbox
| name = GABA receptor modulators
| title = GABA receptor modulators
| state = collapsed
| bodyclass = hlist
| groupstyle = text-align:center;
| group1 = Ionotropic
| list1 = {{Navbox|subgroup
| groupstyle = text-align:center
| groupwidth = 5em
| group1 = GABAA
| list1 =
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ
| list2 =
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
|
|---|
Receptor
(ligands) | | GlyR |
- Positive modulators: Alcohols (e.g., brometone, chlorobutanol (chloretone), ethanol (alcohol), tert-butanol (2M2P), tribromoethanol, trichloroethanol, trifluoroethanol)
- Alkylbenzene sulfonate
- Anandamide
- Barbiturates (e.g., pentobarbital, sodium thiopental)
- Chlormethiazole
- D12-116
- Dihydropyridines (e.g., nicardipine)
- Etomidate
- Ginseng constituents (e.g., ginsenosides (e.g., ginsenoside-Rf))
- Glutamic acid (glutamate)
- Ivermectin
- Ketamine
- Neuroactive steroids (e.g., alfaxolone, pregnenolone (eltanolone), pregnenolone acetate, minaxolone, ORG-20599)
- Nitrous oxide
- Penicillin G
- Propofol
- Tamoxifen
- Tetrahydrocannabinol
- Triclofos
- Tropeines (e.g., atropine, bemesetron, cocaine, LY-278584, tropisetron, zatosetron)
- Volatiles/gases (e.g., chloral hydrate, chloroform, desflurane, diethyl ether (ether), enflurane, halothane, isoflurane, methoxyflurane, sevoflurane, toluene, trichloroethane (methyl chloroform), trichloroethylene)
- Xenon
- Zinc
- Antagonists: 2-Aminostrychnine
- 2-Nitrostrychnine
- 4-Phenyl-4-formyl-N-methylpiperidine
- αEMBTL
- Bicuculline
- Brucine
- Cacotheline
- Caffeine
- Colchicine
- Colubrine
- Cyanotriphenylborate
- Dendrobine
- Diaboline
- Endocannabinoids (e.g., 2-AG, anandamide (AEA))
- Gaboxadol (THIP)
- Gelsemine
- iso-THAZ
- Isobutyric acid
- Isonipecotic acid
- Isostrychnine
- Laudanosine
- N-Methylbicuculline
- N-Methylstrychnine
- N,N-Dimethylmuscimol
- Nipecotic acid
- Pitrazepin
- Pseudostrychnine
- Quinolines (e.g., 4-hydroxyquinoline, 4-hydroxyquinoline-3-carboxylic acid, 5,7-CIQA, 7-CIQ, 7-TFQ, 7-TFQA)
- RU-5135
- Sinomenine
- Strychnine
- Thiocolchicoside
- Tutin
|
|---|
| NMDAR | |
|---|
|
|---|
Transporter
(blockers) | |
|---|
|
|
|---|
| nAChRs | Agonists
(and PAMs) |
- 5-HIAA
- A-84,543
- A-366,833
- A-582,941
- A-867,744
- ABT-202
- ABT-418
- ABT-560
- ABT-894
- Acetylcholine
- Altinicline
- Anabasine
- Anatoxin-a
- AR-R17779
- Bephenium hydroxynaphthoate
- Butinoline
- Butyrylcholine
- Carbachol
- Choline
- Cotinine
- Cytisine
- Decamethonium
- Desformylflustrabromine
- Dianicline
- Dimethylphenylpiperazinium
- Epibatidine
- Epiboxidine
- Ethanol (alcohol)
- Ethoxysebacylcholine
- EVP-4473
- EVP-6124
- Galantamine
- GTS-21
- Ispronicline
- Ivermectin
- JNJ-39393406
- Levamisole
- Lobeline
- MEM-63,908 (RG-3487)
- Morantel
- Nicotine (tobacco)
- NS-1738
- PHA-543,613
- PHA-709,829
- PNU-120,596
- PNU-282,987
- Pozanicline
- Pyrantel
- Rivanicline
- RJR-2429
- Sazetidine A
- SB-206553
- Sebacylcholine
- SIB-1508Y
- SIB-1553A
- SSR-180,711
- Suberyldicholine
- Suxamethonium (succinylcholine)
- Suxethonium (succinyldicholine)
- TC-1698
- TC-1734
- TC-1827
- TC-2216
- TC-5214
- TC-5619
- TC-6683
- Tebanicline
- Tribendimidine
- Tropisetron
- UB-165
- Varenicline
- WAY-317,538
- XY-4083
|
|---|
Antagonists
(and NAMs) | |
|---|
|
|---|
Precursors
(and prodrugs) | |
|---|
|
|
 | Original source: https://en.wikipedia.org/wiki/Laudanosine. Read more |





