Biology:Scopolamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Transdermscop, Kwells, others |
| Other names | Hyoscine[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682509 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal, ophthalmic, subcutaneous, intravenous, sublingual, rectal, buccal, transmucosal, intramuscular |
| Drug class |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 4.5 hours[6] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
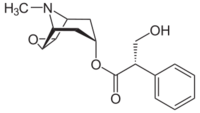
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Scopolamine, also known as hyoscine,[7] or Devil's Breath,[8] is a natural or synthetically produced tropane alkaloid and anticholinergic drug that is used as a medication to treat motion sickness[9] and postoperative nausea and vomiting.[10][1] It is also sometimes used before surgery to decrease saliva.[1] When used by injection, effects begin after about 20 minutes and last for up to 8 hours.[1] It may also be used orally and as a transdermal patch since it has been long known to have transdermal bioavailability.[1][11]
Scopolamine is in the antimuscarinic family of drugs and works by blocking some of the effects of acetylcholine within the nervous system.[1] Scopolamine was first written about in 1881 and started to be used for anesthesia around 1900.[12][13] Scopolamine is also the main active component produced by certain plants of the nightshade family, which historically have been used as psychoactive drugs (known as deliriants) due to their antimuscarinic-induced hallucinogenic effects in higher doses.[10] In these contexts, its mind-altering effects have been utilized for recreational and occult purposes.[14][15][16] The name "scopolamine" is derived from one type of nightshade known as Scopolia, while the name "hyoscine" is derived from another type known as Hyoscyamus niger, or black henbane.[17][18] It is on the World Health Organization's List of Essential Medicines.[19]
Medical uses
Scopolamine has a number of formal uses in modern medicine where it is used in its isolated form and in low doses to treat:[20][21]
- Postoperative nausea and vomiting.
- Motion sickness, including sea sickness, leading to its use by scuba divers (where it is often applied as a transdermal patch behind the ear)[22][23][24][25]
- Gastrointestinal spasms
- Renal or biliary spasms
- Aid in gastrointestinal radiology and endoscopy
- Irritable bowel syndrome
- Clozapine-induced drooling
- Bowel colic
- Eye inflammation[26]
It is sometimes used as a premedication, (especially to reduce respiratory tract secretions) in surgery, most commonly by injection.[20][21] Common side effects include sleepiness, blurred vision, dilated pupils, and dry mouth.[1] It is not recommended in people with angle-closure glaucoma or bowel obstruction.[1] Whether its use during pregnancy is safe remains unclear, and use during breastfeeding is still cautioned by health professionals and manufacturers of the drug.[27]
Breastfeeding
Scopolamine enters breast milk by secretion. Although no human studies exist to document the safety of scopolamine while nursing, the manufacturer recommends that caution be taken if scopolamine is administered to a breastfeeding woman.[27]
Elderly
The likelihood of experiencing adverse effects from scopolamine is increased in the elderly, relative to younger people. This phenomenon is especially true for older people who are also on several other medications. Scopolamine use should be avoided in this age group because of these potent anticholinergic adverse effects, which have also been linked to an increased risk for dementia.[28][29]
Adverse effects
Adverse effect incidence:[5][30][31][32]
Uncommon (0.1–1% incidence) adverse effects include:
- Dry mouth
- Anhidrosis (reduced ability to sweat to cool off)
- Tachycardia (usually occurs at higher doses and is succeeded by bradycardia)
- Bradycardia
- Urticaria (hives)
- Pruritus (itching)
Rare (<0.1% incidence) adverse effects include:
- Constipation
- Urinary retention
- Hallucinations
- Agitation
- Confusion
- Restlessness
- Seizures
Unknown frequency adverse effects include:
- Anaphylactic shock or reactions
- Dyspnea (shortness of breath)
- Rash
- Erythema
- Other hypersensitivity reactions
- Blurred vision
- Mydriasis (dilated pupils)
- Drowsiness
- Dizziness
- Somnolence
- Death
Overdose
Physostigmine, a cholinergic drug that readily crosses the blood–brain barrier, has been used as an antidote to treat the central nervous system depression symptoms of a scopolamine overdose.[33] Other than this supportive treatment, gastric lavage and induced emesis (vomiting) are usually recommended as treatments for oral overdoses.[32] The symptoms of overdose include:[31][32]
- Tachycardia
- Arrhythmia
- Blurred vision
- Photophobia
- Urinary retention
- Drowsiness or paradoxical reaction, which can present with hallucinations
- Cheyne-Stokes respiration
- Dry mouth
- Skin reddening
- Inhibition of gastrointestinal motility
Interactions
Due to interactions with metabolism of other drugs, scopolamine can cause significant unwanted side effects or unpredictable synergies when taken with other medications or compounds. Specific attention should be paid to other medications in the same pharmacologic class as scopolamine, also known as anticholinergics. The following compounds could also potentially interact with the metabolism of scopolamine: receptor-binding analgesic/pain medication such as gabapentinoids or opioids, ethanol, cannabinoids, zolpidem, thiazide diuretics, nicotine, benzodiazepines, buprenorphine, and especially anticholinergic drugs such as tiotropium, diphenhydramine, dimenhydrinate, etc. Nicotine in particular likely has a counteracting effect on the effects of scopolamine due to its opposing effect on acetylcholine signaling.[citation needed]
Route of administration
Scopolamine can be taken by mouth, subcutaneously, in the eye, and intravenously, as well as via a transdermal patch.[34]
Pharmacokinetic
Scopolamine undergoes first-pass metabolism and about 2.6% is excreted unchanged in urine. Grapefruit juice decreases metabolism of scopolamine, consequently increasing plasma concentration.[35]
Pharmacodynamics
The pharmacological effects of scopolamine are mediated through the drug's competitive antagonism of the peripheral and central muscarinic acetylcholine receptors. Scopolamine acts as a nonspecific muscarinic antagonist at all four (M1, M2, M3, and M4) receptor sites.[36][37]
In doses higher than intended for medicinal use; the hallucinogenic alteration of consciousness, as well as the deliriousness in particular are tied to the compound's activity at the M1 muscarinic receptor. M1 receptors are located primarily in the central nervous system and are involved in perception, attention and cognitive functioning. Delirium is only associated with the antagonism of postsynaptic M1 receptors and currently other receptor subtypes have not been implicated.[38] Peripheral muscarinic receptors are part of the autonomic nervous system. M2 receptors are located in the brain and heart, M3 receptors are in salivary glands and M4 receptors are in the brain and lungs.[38] Due to the drug's inhibition of various signal transduction pathways, the decrease in acetylcholine signaling is what leads to many of the cognitive deficits, mental impairments and delirium associated with psychoactive doses. Medicinal effects appear to mostly be tied to activation of the peripheral receptors and only from marginal decreases in acetylcholine signaling.[39]
Although often broadly referred to as simply being 'anticholinergic', antimuscarinic would be more specific and accurate terminology to use for scopolamine, as, for example, it is not known to block nicotinic receptors.[38]
Biosynthesis in plants
Scopolamine is among the secondary metabolites of plants from Solanaceae (nightshade) family of plants, such as henbane (Hyoscyamus niger), jimson weed (Datura), angel's trumpet (Brugmansia), deadly nightshade (Belladonna), mandrake (Mandragora officinarum), and corkwood (Duboisia).[40][17]
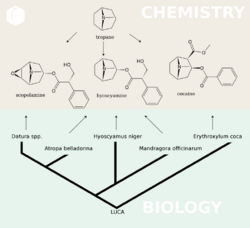
The biosynthesis of scopolamine begins with the decarboxylation of L-ornithine to putrescine by ornithine decarboxylase. Putrescine is methylated to N-methylputrescine by putrescine N-methyltransferase.[41]
A putrescine oxidase that specifically recognizes methylated putrescine catalyzes the deamination of this compound to 4-methylaminobutanal, which then undergoes a spontaneous ring formation to N-methyl-pyrrolium cation. In the next step, the pyrrolium cation condenses with acetoacetic acid yielding hygrine. No enzymatic activity could be demonstrated to catalyze this reaction. Hygrine further rearranges to tropinone.[41]
Subsequently, tropinone reductase I converts tropinone to tropine, which condenses with phenylalanine-derived phenyllactate to littorine. A cytochrome P450 classified as Cyp80F1[42] oxidizes and rearranges littorine to hyoscyamine aldehyde. In the final step, hyoscyamine undergoes epoxidation catalyzed by 6beta-hydroxyhyoscyamine epoxidase yielding scopolamine.[41]
History
Plants naturally containing scopolamine such as Atropa belladonna (deadly nightshade), Brugmansia (angels trumpet), Datura (Jimson weed), Hyoscyamus niger, Mandragora officinarum, Scopolia carniolica, Latua and Duboisia myoporoides have been known about and used for various purposes in both the New and Old Worlds since ancient times.[43][44][45] Being one of the earlier alkaloids isolated from plant sources, scopolamine has been in use in its purified forms (such as various salts, including hydrochloride, hydrobromide, hydroiodide, and sulfate) since its official isolation by the German scientist Albert Ladenburg in 1880,[46] and as various preparations from its plant-based form since antiquity and perhaps prehistoric times. Following the description of the structure and activity of scopolamine by Ladenburg, the search for synthetic analogues, and methods for total synthesis, of scopolamine and atropine in the 1930s and 1940s resulted in the discovery of diphenhydramine, an early antihistamine and the prototype of its chemical subclass of these drugs, and pethidine, the first fully synthetic opioid analgesic, known as Dolantin and Demerol amongst many other trade names.[citation needed]
In 1899, a Dr. Schneiderlin recommended the use of scopolamine and morphine for surgical anaesthesia, and it started to be used sporadically for that purpose.[12][47] The use of this combination in obstetric anesthesiology (childbirth) was first proposed by Richard von Steinbuchel in 1902 and was picked up and further developed by Carl Gauss in Freiburg, Germany , starting in 1903.[48] The method, which was based on a drug synergy between both scopolamine and morphine came to be known as Dämmerschlaf ("twilight sleep") or the "Freiburg method".[47][48] It spread rather slowly, and different clinics experimented with different dosages and ingredients; in 1915, the Canadian Medical Association Journal reported, "the method [was] really still in a state of development".[47] It remained widely used in the US until the 1960s, when growing chemophobia and a desire for more natural childbirth led to its abandonment.[49]
Society and culture
Names
Hyoscine hydrobromide is the international nonproprietary name, and scopolamine hydrobromide is the United States Adopted Name. Other names include levo-duboisine, devil's breath, and burundanga.[15][50]
Australian bush medicine
A bush medicine developed by Aboriginal peoples of the eastern states of Australia from the soft corkwood tree (Duboisia myoporoides) was used by the Allies in World War II to stop soldiers from getting seasick when they sailed across the English Channel on their way to France during the Invasion of Normandy. Later, the same substance was found to be usable in the production of scopolamine and hyoscyamine, which are used in eye surgery, and a multimillion-dollar industry was built in Queensland based on this substance.[51]
Recreational and religious use
While it has been occasionally used recreationally for its hallucinogenic properties, the experiences are often unpleasant, mentally and physically. It is also physically dangerous and officially classified as a deliriant drug, so repeated recreational use is rare.[52] In June 2008, more than 20 people were hospitalized with psychosis in Norway after ingesting counterfeit rohypnol tablets containing scopolamine.[53] In January 2018, 9 individuals were hospitalized in Perth, Western Australia, after reportedly ingesting scopolamine.[54] However, the alkaloid scopolamine, when taken recreationally for its psychoactive effect is usually taken in the form of preparations from plants of the genera Datura or Brugmansia, often by adolescents or young adults in order to achieve hallucinations and an altered state of consciousness induced by muscarinic antagonism.[55][56] In circumstances such as these, the intoxication is usually built on a synergistic, but even more toxic mixture of the additional alkaloids in the plants which includes atropine and hyoscyamine.
Historically, the various plants that produce scopolamine have been used psychoactively for spiritual and magical purposes, particularly by witches in western culture and indigenous groups throughout the Americas such as Native American tribes like the Chumash.[16][57][58][59] When entheogenic preparations of these plants were used, scopolamine was considered to be the main psychoactive compound and was largely responsible for the hallucinogenic effects, particularly when the preparation was made into a topical ointment (most notably flying ointment).[60] Scopolamine is reported to be the only active alkaloid within these plants that can effectively be absorbed through the skin to cause effects.[11] Different recipes for these ointments were explored in European witchcraft at least as far back as the Early Modern period and included multiple ingredients to help with the transdermal absorption of scopolamine (such as animal fat), as well as other possible ingredients to counteract its noxious and dysphoric effects.[60]
In Christianity, although not explicitly designated for ritualistic or spiritual use; in the Bible there are multiple mentions of Mandrake which is a psychoactive and hallucinogenic plant root that contains scopolamine. It was associated with fertility power and (sexual) desire where it was yearned for by Rachel, who apparently was "barren" (infertile) but trying to conceive.[61][62]
Interrogation
The effects of scopolamine were studied for use as a truth serum in interrogations in the early 20th century,[63] but because of the side effects, investigations were dropped.[64] In 2009, the Czechoslovak state security secret police were proven to have used scopolamine at least three times to obtain confessions from alleged antistate dissidents.[65]
Crime in Colombia
A travel advisory published by the US Overseas Security Advisory Council (OSAC) in 2012 stated:
One common and particularly dangerous method that criminals use in order to rob a victim is through the use of drugs. The most common [in Colombia] has been scopolamine. Unofficial estimates put the number of annual scopolamine incidents in Colombia at approximately 50,000. Scopolamine can render a victim unconscious for 24 hours or more. In large doses, it can cause respiratory failure and death. It is most often administered in liquid or powder form in foods and beverages. The majority of these incidents occur in night clubs and bars, and usually men, perceived to be wealthy, are targeted by young, attractive women. It is recommended that, to avoid becoming a victim of scopolamine, a person should never accept food or beverages offered by strangers or new acquaintances, nor leave food or beverages unattended in their presence. Victims of scopolamine or other drugs should seek immediate medical attention.[66]
Between 1998 and 2004, 13% of emergency-room admissions for "poisoning with criminal intentions" in a clinic of Bogotá, Colombia, have been attributed to scopolamine, and 44% to benzodiazepines.[15] Most commonly, the person has been poisoned by a robber who gave the victim a scopolamine-laced beverage, in the hope that the victim would become unconscious or unable to effectively resist the robbery.[15]
Beside robberies, it is also allegedly involved in express kidnappings and sexual assault.[67] The Hospital Clínic in Barcelona introduced a protocol in 2008 to help medical workers identify cases, while Madrid hospitals adopted a similar working document in February 2015.[67] Hospital Clínic has found little scientific evidence to support this use and relies on the victims' stories to reach any conclusion.[67] Although poisoning by scopolamine appears quite often in the media as an aid for raping, kidnapping, killing, or robbery, the effects of this drug and the way it is applied by criminals (transdermal injection, on playing cards and papers, etc.) are often exaggerated,[68][69][70] especially skin exposure, as the dose that can be absorbed by the skin is too low to have any effect.[67] Scopolamine transdermal patches must be used for hours to days.[34] There are certain other aspects of the usage of scopolamine in crimes. Powdered scopolamine is referred to as "devil's breath". In popular media and television, it is portrayed as a method to brainwash or control people into being defrauded by their attackers;[71][72][73][74] there is debate whether these claims are true.[75][76] It is not verified if the powdered form is capable of inducing a suggestive state. The danger is real enough that in addition to the Overseas Security Advisory Council (OSAC) in 2012, the US Department of State, as well as the Government of Canada, published[77][78] travel advisories warning travelers about the possibility of targeting. Criminals using Devil's Breath often use attractive, young women, including women in dating apps[79] to target men that they believe are wealthy.[80] Nevertheless, the drug is known to produce loss of memory following exposure and sleepiness, similar to the effect of benzodiazepines or alcohol poisoning.[81][82]
Research
Scopolamine is used as a research tool to study memory encoding. Initially, in human trials, relatively low doses of the muscarinic receptor antagonist scopolamine were found to induce temporary cognitive defects.[83] Since then, scopolamine has become a standard drug for experimentally inducing cognitive defects in animals.[84][85] Results in primates suggest that acetylcholine is involved in the encoding of new information into long-term memory.[86] Scopolamine has also been shown to exert a greater impairment on episodic memory, event-related potentials, memory retention and free recall compared to DPH (an anticholinergic and antihistamine).[82]
Scopolamine produces detrimental effects on short-term memory, memory acquisition, learning, visual recognition memory, visuospatial praxis, visuospatial memory, visuoperceptual function, verbal recall, and psychomotor speed.[87][84][85] It does not seem to impair recognition and memory retrieval, though.[85] Acetylcholine projections in hippocampal neurons, which are vital in mediating long-term potentiation, are inhibited by scopolamine.[85][88] Scopolamine also inhibits cholinergic-mediated glutamate release in hippocampal neurons, which assist in depolarization, potentiation of action potential, and synaptic suppression. Scopolamine's effects on acetylcholine and glutamate release in the hippocampus favor retrieval-dominant cognitive functioning.[85] Scopolamine has been used to model the defects in cholinergic function for models of Alzheimer's, dementia, fragile X syndrome, and Down syndrome.[85][89][90][91]
Scopolamine has been identified as a psychoplastogen, which refers to a compound capable of promoting rapid and sustained neuroplasticity in a single dose.[92] It has been, and continues to be investigated as a rapid-onset antidepressant, with a number of small studies finding positive results, particularly in female subjects.[93][94][95][96]
NASA agreed to develop a nasal administration method. With a precise dosage, the NASA spray formulation has been shown to work faster and more reliably than the oral form to treat motion sickness.[97]
Although a fair amount of research has been applied to scopolamine in the field of medicine, its hallucinogenic (psychoactive) effects as well as the psychoactive effects of other antimuscarinic deliriants haven't been extensively researched or as well understood compared to other types of hallucinogens such as psychedelic and disassociative compounds, despite the alkaloid's long history of usage in mind-altering plant preparations.[98]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Scopolamine". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/scopolamine.html.
- ↑ "Poisons Standard October 2020". 30 September 2020. https://www.legislation.gov.au/Details/F2020L01255.
- ↑ "Hyoscine Hydrobromide 400 micrograms/ml Solution for Injection - Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/9655/smpc.
- ↑ "Kwells 300 microgram tablets - Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/250/smpc.
- ↑ 5.0 5.1 "Transderm Scop- scopolamine patch, extended release". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7192d89d-ea4b-42b0-b8c1-e7fd41be8a0a.
- ↑ "Pharmacokinetics and oral bioavailability of scopolamine in normal subjects". Pharmaceutical Research 06 (6): 481–485. June 1989. doi:10.1023/A:1015916423156. PMID 2762223.
- ↑ Concise Dictionary of Biomedicine and Molecular Biology. (2nd ed.). Hoboken: CRC Press. 2001. p. 570. ISBN 9781420041309. https://books.google.com/books?id=Y4DLBQAAQBAJ&pg=PA570.
- ↑ "Colombian Devil's Breath". 23 July 2007. https://www.vice.com/en/article/kw3kam/colombian-devil-s-breath-1-of-2.
- ↑ "About hyoscine hydrobromide" (in en). 2022-10-24. https://www.nhs.uk/medicines/hyoscine-hydrobromide/about-hyoscine-hydrobromide/.
- ↑ 10.0 10.1 Plant-derived Natural Products: Synthesis, Function, and Application. Springer Science & Business Media. 2009. p. 5. ISBN 9780387854984. https://books.google.com/books?id=Y8SpVXEng4QC&pg=PA6.
- ↑ 11.0 11.1 A Manual of Pharmacology and Its Applications to Therapeutics and Toxicology (8th ed.). Philadelphia and London: W.B. Saunders. 1957.
- ↑ 12.0 12.1 The history of surgical anesthesia (Reprint ed.). Park Ridge, Ill.: Wood Library, Museum of Anesthesiology. 1996. p. 48ff. ISBN 978-0-9614932-7-1. https://www.woodlibrarymuseum.org/wp-content/uploads/e-books/w0012pdf.PDF.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 551. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA551.
- ↑ "The Deliriants - The Nightshade (Solanaceae) Family". Plants and the Human Brain. New York City: Oxford University Press. 2014. pp. 131–137. ISBN 9780199914012. https://books.google.com/books?id=YUNDAgAAQBAJ&pg=PA131. Retrieved 2021-09-17.
- ↑ 15.0 15.1 15.2 15.3 "Perfil epidemiológico de la intoxicación con burundanga en la clínica Uribe Cualla S. A. de Bogotá, D. C" (in es). Acta Neurológica Colombiana 21 (3): 197–201. September 2005. http://www.acnweb.org/acta/2005_21_3_197.pdf.
- ↑ 16.0 16.1 The encyclopedia of psychoactive plants: ethnopharmacology and its applications. US: Park Street Press. 2005. pp. 277–282.
- ↑ 17.0 17.1 The Chambers Dictionary. Allied Publishers. 1998. pp. 788, 1480. ISBN 978-81-86062-25-8.
- ↑ Lippincott's new medical dictionary: a vocabulary of the terms used in medicine, and the allied sciences, with their pronunciation, etymology, and signification, including much collateral information of a descriptive and encyclopedic character. Lippincott. 1910. p. 435. https://books.google.com/books?id=W_hEAAAAQAAJ&pg=PA435. Retrieved 25 February 2012.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 20.0 20.1 Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 49, 266, 822, 823. ISBN 978-0-85711-084-8. https://archive.org/details/bnf65britishnati0000unse/page/49.
- ↑ 21.0 21.1 Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ↑ "Hyperbaric oxygen and scopolamine". Undersea Biomedical Research 18 (3): 167–174. May 1991. PMID 1853467. http://archive.rubicon-foundation.org/2573. Retrieved 13 August 2008.
- ↑ "Effects of transcutaneous scopolamine and depth on diver performance". Undersea Biomedical Research 15 (2): 89–98. March 1988. PMID 3363755. http://archive.rubicon-foundation.org/2495.
- ↑ "Effects of transcutaneous scopolamine and depth on diver performance". Undersea Biomedical Research 15 (2): 89–98. March 1988. PMID 3363755.
- ↑ "Motion Sickness". https://dan.org/health-medicine/health-resources/diseases-conditions/motion-sickness/.
- ↑ "scopolamine solution - ophthalmic, Isopto". https://www.medicinenet.com/scopolamine_drops-ophthalmic/article.htm.
- ↑ 27.0 27.1 "Scopolamine". Drugs in Pregnancy and Lactation. Baltimore, Maryland: Williams and Wilkins. 1994. pp. 777–778. ISBN 9780683010602. https://archive.org/details/drugsinpregnancy00gera/page/777.
- ↑ "Study suggests link between long-term use of anticholinergics and dementia risk". Alzheimer's Society. 26 January 2015. http://www.alzheimers.org.uk/site/scripts/news_article.php?newsID=2300.
- ↑ "Hypersensitivity to scopolamine in the elderly". Psychopharmacology 107 (2–3): 437–441. 1992. doi:10.1007/bf02245172. PMID 1615141.
- ↑ "DBL HYOSCINE INJECTION BP". TGA eBusiness Services. Hospira Australia Pty Ltd. 30 January 2012. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2009-PI-00398-3.
- ↑ 31.0 31.1 "Buscopan Tablets - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Boehringer Ingelheim Limited. 11 September 2013. http://www.medicines.org.uk/emc/medicine/282/SPC/Buscopan+Tablets/.
- ↑ 32.0 32.1 32.2 "Kwells 300 microgram tablets - Summary of Product Characteristics". electronic Medicines Compendium. Bayer plc. 7 January 2008. http://www.medicines.org.uk/emc/medicine/18845/SPC/Kwells+300+microgram+tablets/.
- ↑ Clinical anesthesia (6th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. 2009. p. 346. ISBN 978-0-7817-8763-5.
- ↑ 34.0 34.1 "Transdermal scopolamine: an alternative to ondansetron and droperidol for the prevention of postoperative and postdischarge emetic symptoms". Anesthesia and Analgesia 104 (1): 92–96. January 2007. doi:10.1213/01.ane.0000250364.91567.72. PMID 17179250.
- ↑ "Pharmacokinetics and pharmacodynamics in clinical use of scopolamine". Therapeutic Drug Monitoring 27 (5): 655–665. October 2005. doi:10.1097/01.ftd.0000168293.48226.57. PMID 16175141.
- ↑ "Google Scholar". https://scholar.google.com/scholar?q=scopolamine+nonselective&btnG=&hl=en&as_sdt=0%2C11.
- ↑ "PDSP Ki Database". https://pdsp.unc.edu/databases/pdsp.php?receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testDDRadio=testDDRadio&testLigandDD=2417&testLigand=&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query.
- ↑ 38.0 38.1 38.2 "Pharmacological management of anticholinergic delirium - theory, evidence and practice". British Journal of Clinical Pharmacology 81 (3): 516–524. March 2016. doi:10.1111/bcp.12839. PMID 26589572. "Delirium is only associated with the antagonism of post‐synaptic M1 receptors and to date other receptor subtypes have not been implicated".
- ↑ "Abuse of prescription and over-the-counter medications". Journal of the American Board of Family Medicine 21 (1): 45–54. 2008. doi:10.3122/jabfm.2008.01.070071. PMID 18178702.
- ↑ "Continuous Production of Scopolamine by a Culture of Duboisia leichhardtii Hairy Root Clone in a Bioreactor System". Applied Microbiology and Biotechnology 40 (2–3): 219–223. 1993. doi:10.1007/BF00170370.
- ↑ 41.0 41.1 41.2 "Alkaloid biosynthesis: metabolism and trafficking". Annual Review of Plant Biology 59 (1): 735–769. 2008. doi:10.1146/annurev.arplant.59.032607.092730. PMID 18251710.
- ↑ "Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement". Chemistry & Biology 13 (5): 513–520. May 2006. doi:10.1016/j.chembiol.2006.03.005. PMID 16720272.
- ↑ Armando T. Hunziker: The Genera of Solanaceae. A.R.G. Gantner Verlag K.G., Ruggell, Liechtenstein 2001. ISBN:3-904144-77-4
- ↑ Rätsch, Christian, The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications pub. Park Street Press 2005
- ↑ "Pharmacognosy and Phytochemistry : Drugs Containing Alkaloids". http://www.pharmacy180.com/article/duboisia-131/#:~:text=Chemical%20Constituents,%2C%20tigloidine%2C%20valtropine%2C%20tiglyoxytropine..
- ↑ "Die natürlich vorkommenden mydriatisch wirkenden Alkaloïde" (in de). Annalen der Chemie 206 (3): 274–307. 1880. doi:10.1002/jlac.18812060303. https://babel.hathitrust.org/cgi/pt?id=hvd.hx3khy;view=1up;seq=690.; see pp. 299–307.
- ↑ 47.0 47.1 47.2 "Twilight Sleep: the Dammerschlaf of the Germans". Canadian Medical Association Journal 5 (9): 805–808. September 1915. PMID 20310688.
- ↑ 48.0 48.1 "TWILIGHT SLEEP; Is Subject of a New Investigation". The New York Times. 31 January 1915. https://query.nytimes.com/gst/abstract.html?res=9E05EED8113EE733A05752C3A9679C946496D6CF.
- ↑ "Labor Dispute. Book review: What a Blessing She Had Chloroform: The Medical and Social Response to the Pain of Childbirth from 1800 to the Present". The New York Times. 31 October 1999. https://www.nytimes.com/1999/10/31/books/labor-dispute.html.
- ↑ "Mind controller: What is the 'burundanga' drug?". Wired UK. 3 March 2011. April 2011. https://www.wired.co.uk/article/mind-controller-1.
- ↑ "Visitors to Art of Healing exhibition told how Australian Indigenous bush medicine was given to every allied soldier landing at Normandy on D-Day". 7 June 2019. https://www.kcl.ac.uk/news/australian-indigenous-bush-medicine-was-given-to-allied-soldiers-on-d-day.
- ↑ "Toxicity of Datura Stramonium". Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs. Netherlands: Springer. 2010. pp. 217–218. doi:10.1007/978-90-481-2448-0_34. ISBN 978-90-481-2447-3.
- ↑ "Bilsykemedisin i falske rohypnol-tabletter". http://www.aftenposten.no/nyheter/iriks/article2507100.ece.
- ↑ "Perth backpacker overdose linked to common anti-nausea drug". ABC News. 4 January 2018. http://www.abc.net.au/news/2018-01-04/toxicology-results-from-perth-mass-overdose-expected-today/9303330.
- ↑ "Common anticholinergic solanaceaous plants of temperate Europe - A review of intoxications from the literature (1966-2018)". Toxicon 177: 52–88. April 2020. doi:10.1016/j.toxicon.2020.02.005. PMID 32217234.
- ↑ Brugmansia and Datura: Angel's Trumpets and Thorn Apples. Buffalo, NY: Firefly Books. 2002. pp. 106–129. ISBN 1-55209-598-3.
- ↑ The Way of the Shaman. New York: Harper & Row. 1980. ISBN 9780062503732. https://archive.org/details/wayofshamanguide00harn.
- ↑ How Do Witches Fly?. DNA Press. February 1999. ISBN 0-9664027-0-7.
- ↑ "The Datura Cult Among the Chumash; The Journal of California Anthropology". https://escholarship.org/content/qt37r1g44r/qt37r1g44r.pdf?t=krnkzn.
- ↑ 60.0 60.1 Hansen, Harold A. The Witch's Garden pub. Unity Press 1978 ISBN:978-0913300473
- ↑ "Genesis 30:14–16 (King James Version)". Bible Gateway. http://www.biblegateway.com/passage/?search=Genesis%2030:14-16&version=KJV.
- ↑ "Song of Songs 7:12–13 (King James Version)". Bible Gateway. http://www.biblegateway.com/passage/?search=Song%20of%20Songs%207:12-13&version=KJV.
- ↑ "The Use of Scopolamine in Criminology". Texas State Journal of Medicine 18: 256–263. September 1922.
Reprinted in: "The Use of Scopolamine in Criminology". American Journal of Police Science 2 (4): 328–336. July–August 1931. doi:10.2307/1147361. - ↑ "'Truth' Drugs in Interrogation". Central Intelligence Agency. 22 September 1993. https://www.cia.gov/library/center-for-the-study-of-intelligence/kent-csi/vol5no2/html/v05i2a09p_0001.htm.
- ↑ "Svědek: Grebeníček vězně nejen mlátil, ale dával jim i drogy" (in cs). iDnes. 8 August 2009. http://zpravy.idnes.cz/svedek-grebenicek-vezne-nejen-mlatil-ale-daval-jim-i-drogy-pmd-/domaci.asp?c=A090807_205833_domaci_vel.
- ↑ "Colombia 2012 Crime and Safety Report: Cartagena". Overseas Security Advisory Council, United States Department of State. 4 March 2012. https://www.osac.gov/Pages/ContentReportDetails.aspx?cid=12118.
- ↑ 67.0 67.1 67.2 67.3 "Burundanga: the stealth drug that cancels the victim's willpower". Crime. El País, Madrid. 25 July 2016. http://elpais.com/elpais/2016/07/25/inenglish/1469445136_776085.html?id_externo_promo=ob-externo-english.
- ↑ "Burundanga Business Card Drug Warning". 12 October 2008. http://www.hoax-slayer.com/burundanga-warning.shtml.
- ↑ "Beware the Burundanga Man!". About.com Entertainment. http://urbanlegends.about.com/od/crime/a/burundanga.htm.
- ↑ "Burundanga/Scopolamine Warning". snopes.com. http://www.snopes.com/crime/warnings/burundanga.asp.
- ↑ "Million dollar ride: Crime committed during involuntary scopolamine intoxication". Canadian Family Physician 63 (5): 369–370. May 2017. PMID 28500194. PMC 5429053. https://www.cfp.ca/content/cfp/63/5/369.full.pdf.
- ↑ "World's Scariest Drug (Documentary Exclusive)" (in en). https://www.youtube.com/watch?v=ToQ8PWYnu04.
- ↑ "أخطر مخدر في العالم (عدنا إلى كولومبيا)" (in ar). https://www.youtube.com/watch?v=iuPnouyasN4.
- ↑ "المخدر الأخطر في العالم ستتعرض له بدون أن تشعر في أمريكا اللاتينية" (in ar). https://www.youtube.com/watch?v=wh8RVl6uA4I&t=97s.
- ↑ "Devil's Breath: Urban Legend or the World's Most Scary Drug?". https://www.drugs.com/illicit/devils-breath.html.
- ↑ "'Devil's breath' aka scopolamine: can it really zombify you?". The Guardian. 2 September 2015. https://www.theguardian.com/society/shortcuts/2015/sep/02/devils-breath-aka-scopolamine-can-it-really-zombify-you.
- ↑ "Colombia Travel Advisory". https://travel.state.gov/content/travel/en/traveladvisories/traveladvisories/colombia-travel-advisory.html.
- ↑ Global Affairs Canada (16 November 2012). "Travel Advice and Advisories for Colombia". https://travel.gc.ca/destinations/colombia.
- ↑ https://www.semana.com/amp/salud/articulo/escopolamina-la-droga-borra-recuerdos-que-acecha-a-los-extranjeros-en-colombia-estos-son-sus-mortales-efectos/202423/
- ↑ "Devil's Breath: Why Scopolamine Abuse is So Terrifying". 9 May 2019. https://www.northpointwashington.com/blog/devils-breath-scopolamine-abuse-terrifying/.
- ↑ "Atypical Drugs of Abuse". Articles & Interviews. Student Doctor Network. 27 July 2008. http://studentdoctor.net/2008/07/atypical-drugs-of-abuse/.
- ↑ 82.0 82.1 "Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazepam and diphenhydramine". Psychopharmacology 135 (1): 27–36. January 1998. doi:10.1007/s002130050482. PMID 9489931.
- ↑ "Human memory and the cholinergic system. A relationship to aging?". Archives of Neurology 30 (2): 113–121. February 1974. doi:10.1001/archneur.1974.00490320001001. PMID 4359364.
- ↑ 84.0 84.1 "Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function". Behavioural Brain Research 89 (1–2): 1–34. December 1997. doi:10.1016/s0166-4328(97)00048-x. PMID 9475612.
- ↑ 85.0 85.1 85.2 85.3 85.4 85.5 "Toxin-Induced Experimental Models of Learning and Memory Impairment". International Journal of Molecular Sciences 17 (9): 1447. September 2016. doi:10.3390/ijms17091447. PMID 27598124.
- ↑ "An involvement of acetylcholine in object discrimination learning and memory in the marmoset". Neuropsychologia 22 (3): 253–263. 1984. doi:10.1016/0028-3932(84)90073-3. PMID 6431311.
- ↑ "Scopolamine effects on memory, language, visuospatial praxis and psychomotor speed". Psychopharmacology 100 (2): 243–250. February 1990. doi:10.1007/bf02244414. PMID 2305013.
- ↑ "Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies". Psychopharmacology 236 (1): 201–226. January 2019. doi:10.1007/s00213-018-5127-x. PMID 30604182.
- ↑ "Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome". Behavioural Brain Research 291: 164–171. September 2015. doi:10.1016/j.bbr.2015.05.003. PMID 25979787.
- ↑ "Neurological phenotypes for Down syndrome across the life span". Down Syndrome: From Understanding the Neurobiology to Therapy. Progress in Brain Research. 197. 2012. pp. 101–21. doi:10.1016/b978-0-444-54299-1.00006-6. ISBN 9780444542991.
- ↑ "Anandamides inhibit binding to the muscarinic acetylcholine receptor". Journal of Molecular Neuroscience 13 (1–2): 55–61. 1999. doi:10.1385/jmn:13:1-2:55. PMID 10691292.
- ↑ "Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics". Journal of Experimental Neuroscience 12: 1179069518800508. September 19, 2018. doi:10.1177/1179069518800508. PMID 30262987.
- ↑ "Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review". Biological Psychiatry 73 (12): 1156–1163. June 2013. doi:10.1016/j.biopsych.2012.09.031. PMID 23200525.
- ↑ "Scopolamine and depression: a role for muscarinic antagonism?". CNS & Neurological Disorders Drug Targets 13 (4): 673–683. 2014. doi:10.2174/1871527313666140618105710. PMID 24938776.
- ↑ "Scopolamine as an antidepressant: a systematic review". Clinical Neuropharmacology 36 (1): 24–26. 2013. doi:10.1097/wnf.0b013e318278b703. PMID 23334071.
- ↑ "GABA interneurons mediate the rapid antidepressant-like effects of scopolamine". The Journal of Clinical Investigation 126 (7): 2482–2494. July 2016. doi:10.1172/JCI85033. PMID 27270172.
- ↑ "NASA Signs Agreement to Develop Nasal Spray for Motion Sickness". NASA (Press release). 12 October 2012. Retrieved 3 February 2022.[yes|permanent dead link|dead link}}]
- ↑ "Understanding Central Nervous System Effects of Deliriant Hallucinogenic Drugs through Experimental Animal Models". ACS Chemical Neuroscience 10 (1): 143–154. January 2019. doi:10.1021/acschemneuro.8b00433. PMID 30252437.
External links
- "Scopolamine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/scopolamine.
 |


