Chemistry:Befiradol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
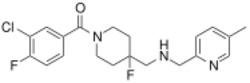
| Formula | C20H22ClF2N3O |
| Molar mass | 393.86 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Befiradol (F-13,640; NLX-112) is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
Pharmacology
In recombinant cell lines expressing human 5-HT1A receptors, befiradol exhibits high agonist efficacy for a variety of signal transduction read-outs, including ERK phosphorylation, G-protein activation, receptor internalization and adenylyl cyclase inhibition.[1] In rat hippocampal membranes it preferentially activates GalphaO proteins.[1] In neurochemical experiments, befiradol activated 5-HT1A autoreceptors in rat dorsal Raphe nucleus as well as 5-HT1A heteroreceptors on pyramidal neurons in the frontal cortex.[2] In rat models, it has powerful analgesic and antiallodynic effects comparable to those of high doses of opioid painkillers, but with fewer and less prominent side effects, as well as little or no development of tolerance with repeated use.[3][4][5][6][7]
A structure–activity relationship (SAR) study revealed that replacement of the dihalophenyl moiety by 3-benzothienyl increases maximal efficacy from 84% to 124% (Ki=2.7 nM).[8][9]
History
Befiradol was discovered and developed by Pierre Fabre Médicament, a French pharmaceuticals company who initially developed it as a treatment for chronic pain. In September 2013, befiradol was out-licensed to Neurolixis, a US-based biotechnology company. Neurolixis announced that it intended to re-purpose befiradol for the treatment of levodopa-induced dyskinesia in Parkinson's disease.[10] In support of this indication, Neurolixis received several research grants[11] from the Michael J. Fox Foundation and preclinical data was published describing the activity of befiradol in animal models of Parkinson's disease.[12][13] Studies published in 2020 using non-human primate models of Parkinson's disease, (MPTP-treated marmosets and MPTP-treated macaques), found that befiradol potently reduced Levodopa-induced dyskinesia at oral doses as low as 0.1 to 0.4 mg/kg.[14][15] In January 2018, the British charity Parkinson's UK announced that it had awarded Neurolixis a grant to advance development of befiradol up to clinical phase in Parkinson's disease patients.[16]
Clinical Ph2A Trial for dyskinesia in Parkinson's disease
In March 2019, Neurolixis announced that the US Food and Drug Administration (FDA) gave a positive response to Neurolixis' Investigational New Drug (IND) application for NLX-112 to be tested in a Phase 2 clinical study in Parkinson's disease patients with troublesome levodopa-induced dyskinesia.[17] On 22 November 2020, The Sunday Times reported that the two charities, Parkinson's UK and Michael J. Fox Foundation, were jointly investing $2 million to support a clinical trial on befiradol in Parkinson's disease patients with troublesome Levodopa-induced dyskinesia, a potentially "life changing" drug.[18] On 23 November 2020, Parkinson's UK and Michael J. Fox Foundation, confirmed their funding in an official announcement.[19] Neurolixis announced on 30 November 2021 the start of patient recruitment in the clinical trial. The trial is listed on the U.S. National Library of Medicine clinical trials register.[20] On 20 March 2023, a joint press release from Neurolixis, Parkinson's UK and Michael J. Fox Foundation announced that the clinical trial had met its primary endpoint of safety and tolerability, and also the secondary endpoint of efficacy in reducing Levodopa-induced dyskinesia in the patients.[21] Moreover, a later announcement (7 July 2023) disclosed that the clinical trial had also found that befiradol reduced parkinsonism symptoms (such as slowness of movement, tremor and rigidity), as well as Levodopa-induced dyskinesia, raising the prospect of developing a "dual-efficacy therapy" for Parkinson's disease.[22]
18F-Befiradol as an agonist PET radiotracer for brain imaging
As well as studies on befiradol for treatment of movement disorders, other researchers have investigated it as a novel radiotracer for brain imaging studies by positron emission tomography. Thus befiradol labeled with [18F] (also known as 18F-F13640) has been used to study the distribution of serotonin 5-HT1A receptors in rat, cat, macaque and human. Because befiradol is an agonist, it enables the detection of 5-HT1A receptors which are specifically in a functionally active state, whereas antagonist radiotracers label the total receptor population.[23][24]
See also
References
- ↑ 1.0 1.1 "Distinctive in vitro signal transduction profile of NLX-112, a potent and efficacious serotonin 5-HT1A receptor agonist". The Journal of Pharmacy and Pharmacology 69 (9): 1178–1190. September 2017. doi:10.1111/jphp.12762. PMID 28612503.
- ↑ "In vivo electrophysiological and neurochemical effects of the selective 5-HT1A receptor agonist, F13640, at pre- and postsynaptic 5-HT1A receptors in the rat". Psychopharmacology 221 (2): 261–272. May 2012. doi:10.1007/s00213-011-2569-9. PMID 22147258.
- ↑ "Profound, non-opioid analgesia produced by the high-efficacy 5-HT(1A) agonist F 13640 in the formalin model of tonic nociceptive pain". Pharmacology 67 (4): 182–194. April 2003. doi:10.1159/000068404. PMID 12595749.
- ↑ "Tolerance and inverse tolerance to the hyperalgesic and analgesic actions, respectively, of the novel analgesic, F 13640". European Journal of Pharmacology 466 (3): 271–279. April 2003. doi:10.1016/S0014-2999(03)01566-8. PMID 12694810.
- ↑ "Dual, hyperalgesic, and analgesic effects of the high-efficacy 5-hydroxytryptamine 1A (5-HT1A) agonist F 13640 [(3-chloro-4-fluoro-phenyl)-[4-fluoro-4-{[(5-methyl-pyridin-2-ylmethyl)-amino]-methyl}piperidin-1-yl]methanone, fumaric acid salt]: relationship with 5-HT1A receptor occupancy and kinetic parameters". The Journal of Pharmacology and Experimental Therapeutics 312 (3): 1034–1042. March 2005. doi:10.1124/jpet.104.077669. PMID 15528450.
- ↑ "High-efficacy 5-hydroxytryptamine 1A receptor activation counteracts opioid hyperallodynia and affective conditioning". The Journal of Pharmacology and Experimental Therapeutics 316 (2): 892–899. February 2006. doi:10.1124/jpet.105.095109. PMID 16254131.
- ↑ "Curative-like analgesia in a neuropathic pain model: parametric analysis of the dose and the duration of treatment with a high-efficacy 5-HT(1A) receptor agonist". European Journal of Pharmacology 568 (1–3): 134–141. July 2007. doi:10.1016/j.ejphar.2007.04.022. PMID 17512927.
- ↑ "Novel pyridylmethylamines as highly selective 5-HT(1A) superagonists". Journal of Medicinal Chemistry 53 (19): 7167–7179. October 2010. doi:10.1021/jm100835q. PMID 20860381.
- ↑ "Novel derivatives of 2-pyridinemethylamine as selective, potent, and orally active agonists at 5-HT1A receptors". Journal of Medicinal Chemistry 42 (9): 1648–1660. May 1999. doi:10.1021/jm9806906. PMID 10229633.
- ↑ "Neurolixis Announces In-Licensing of Two Clinical Compounds From Pierre Fabre Medicament". Neurolixis, Inc.. 23 September 2013. http://neurolixis.com/images/stories/nlx_pf_license_23sept13.pdf.
- ↑ "Parkinson's Disease Grants funded by the Michael J. Fox Foundation | Parkinson's Disease". https://www.michaeljfox.org/foundation/funded-grants.php?srch=Neurolixis&x=0&y=0&from=2000&to=&country=&institution=&researcher=.
- ↑ "NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: Behavioral and neurochemical profile in rat". Experimental Neurology 271: 335–350. September 2015. doi:10.1016/j.expneurol.2015.05.021. PMID 26037043.
- ↑ "The novel 5-HT1A receptor agonist, NLX-112 reduces l-DOPA-induced abnormal involuntary movements in rat: A chronic administration study with microdialysis measurements". Neuropharmacology 105: 651–660. June 2016. doi:10.1016/j.neuropharm.2016.01.013. PMID 26777281.
- ↑ "The selective 5-HT1A receptor agonist, NLX-112, exerts anti-dyskinetic effects in MPTP-treated macaques". Parkinsonism & Related Disorders 78: 151–157. September 2020. doi:10.1016/j.parkreldis.2020.08.009. PMID 32846366.
- ↑ "The selective 5-HT1A receptor agonist, NLX-112, exerts anti-dyskinetic and anti-parkinsonian-like effects in MPTP-treated marmosets". Neuropharmacology 167: 107997. May 2020. doi:10.1016/j.neuropharm.2020.107997. PMID 32057799.
- ↑ "Investing in a new treatment for dyskinesia". Parkinson's UK. 24 January 2018. https://www.parkinsons.org.uk/news/investing-new-treatment-dyskinesia.
- ↑ "FDA Approves Neurolixis IND Application for a Clinical Trial with NLX-112 in Parkinson's Disease". Neurolixis, Inc.. 12 March 2019. https://www.prlog.org/12758787-fda-approves-neurolixis-ind-application-for-clinical-trial-with-nlx-112-in-parkinsons-disease.html.
- ↑ "'Life-changing' drug to calm Parkinson's twitches set for human trials". Thetimes.co.uk. 22 November 2020. https://www.thetimes.co.uk/article/life-changing-drug-to-calm-parkinsons-twitches-set-for-human-trials-pj6xqxfg7.
- ↑ "Global charities join forces to drive forward new drug for Parkinson's". The Michael J. Fox Foundation for Parkinson's Research (Press release) – via Cision US Inc.
- ↑ Clinical trial number NCT05148884 for "Study to Assess the Safety, Tolerability and Preliminary Efficacy of NLX-112 Versus Placebo in L-dopa-induced Dyskinesia" at ClinicalTrials.gov
- ↑ "Neurolixis Announces Positive Ph2A Proof-of-Concept on NLX-112 in Levodopa-Induced Dyskinesia in Parkinson's Disease". 20 March 2023. https://www.einnews.com/pr_news/622375270/neurolixis-announces-positive-ph2a-proof-of-concept-on-nlx-112-in-levodopa-induced-dyskinesia-in-parkinson-s-disease.
- ↑ Massey, Nina (7 July 2023). "Researchers hopeful of treatment of Parkinson's by 2030 with 'dual efficacy' drug". The Independent. https://www.independent.co.uk/news/health/parkinsons-disease-drug-treatment-trial-b2370993.html.
- ↑ "[18FF13640, a 5-HT1A Receptor Radiopharmaceutical Sensitive to Brain Serotonin Fluctuations"]. Frontiers in Neuroscience 15: 622423. 2021. doi:10.3389/fnins.2021.622423. PMID 33762906.
- ↑ "[18FF13640: a selective agonist PET radiopharmaceutical for imaging functional 5-HT1A receptors in humans"]. European Journal of Nuclear Medicine and Molecular Imaging 50 (6): 1651–1664. May 2023. doi:10.1007/s00259-022-06103-1. PMID 36656363.
 |

