Chemistry:Promegestone
 | |
| Clinical data | |
|---|---|
| Trade names | Surgestone |
| Other names | PMG; R-5020; RU-5020; 17α,21-Dimethyl-δ9-19-norprogesterone; 17α,21-Dimethyl-19-norpregna-4,9-diene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | To albumin[1] |
| Metabolism | Liver (hydroxylation)[1][3] |
| Metabolites | • Trimegestone |
| Elimination half-life | Promegestone: ? Trimegestone: 13.8–15.6 hours[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H30O2 |
| Molar mass | 326.480 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders.[4][1][5][6] It is taken by mouth.[1]
Side effects of promegestone include menstrual irregularities among others.[7] Promegestone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has weak antiandrogenic, glucocorticoid, and antimineralocorticoid activity and no other important hormonal activity.[1][8][2] The medication is largely a prodrug of trimegestone.[7][1]
Promegestone was first described in 1973 and was introduced for medical use in France in 1983.[9][10][11] It has only been marketed in a few countries, including France, Portugal, Tunisia, and Argentina .[6][12] In addition to its use as a medication, promegestone has been widely used in scientific research as a radioligand of the progesterone receptor.[4][13]
Medical uses
Promegestone is used in menopausal hormone therapy and in the treatment of gynecological conditions caused by luteal insufficiency, including premenopausal disorders, dysmenorrhea and other menstrual disorders, and premenstrual syndrome.[1][5] It has also been used to treat benign breast disorders such as mastalgia (breast pain).[14] Promegestone tablets have a contraceptive effect and are used as a form of progestogen-only birth control, although it is not specifically licensed as such.[15]
Side effects
Side effects of promegestone include menstrual irregularities among others.[7] It has no androgenic side effects.[4][5]
Pharmacology
Pharmacodynamics
Promegestone is a progestogen, or an agonist of the progesterone receptor.[1][3] It has about 200% of the affinity of progesterone for the PR.[1][3] The endometrial transformation dosage of promegestone is 10 mg per cycle and its ovulation-inhibiting dosage is 0.5 mg/day.[1][3] Promegestone has weak glucocorticoid activity in addition to its progestogenic activity.[1][3] Conversely, it has no androgenic, estrogenic, mineralocorticoid, or other hormonal activity.[1][3][5] It appears to possess antiandrogenic activity.[13] Its major metabolite trimegestone has weak antimineralocorticoid and antiandrogenic activity.[8][2] In addition, promegestone has been found to possess some neurosteroid activity by acting as a non-competitive antagonist of the nicotinic acetylcholine receptor, similarly to progesterone.[16]
Pharmacokinetics
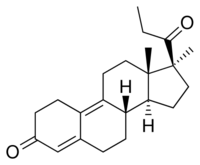
Following oral administration, peak serum levels of promegestone are reached after 1 to 2 hours.[1][3] The medication is mainly bound to albumin; it does not bind to sex hormone-binding globulin, and binds only weakly to corticosteroid-binding globulin.[1][3][17] The metabolism of promegestone is mainly via hydroxylation at the C21 position and at other positions.[1][3] Progesterone is similarly hydroxylated at the C21 position, into 11-deoxycorticosterone (21-hydroxyprogesterone).[18] However, the C9(10) double bond of promegestone greatly limits the A-ring reduction that progesterone undergoes, resulting in 21-hydroxylation being the main route of metabolism for promegestone.[18] The medication is stereoselectively metabolized into trimegestone, the 21(S)-hydroxy metabolite, which is the main compound found in plasma; it circulates at levels approximately twice those of promegestone itself.[7] In addition, trimegestone has more than three-fold higher affinity for the PR than does promegestone.[1] As such, promegestone is largely a prodrug of trimegestone.[7][19] A second metabolite, 21(R)-hydroxypromegestone, circulates at far lower concentrations (AUC ratio for the (S)- and (R)-isomers of about 21).[7] The elimination half-life of trimegestone is 13.8 to 15.6 hours.[1][2] Promegestone, trimegestone, and 21(R)-hydroxypromegestone are not excreted in urine, while 3% of a dose is recovered as the glucuronide and/or sulfate conjugate of trimegestone and 1% of a dose is recovered as the glucuronide and/or sulfate conjugate of 21(R)-hydroxypromegestone.[7]
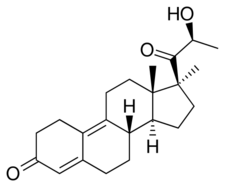
Chemistry
Promegestone, also known as 17α,21-dimethyl-δ9-19-norprogesterone or as 17α,21-dimethyl-19-norpregna-4,9-diene-3,20-dione, is a synthetic norpregnane steroid and a derivative of progesterone.[9][12][11][1] It is specifically a combined derivative of 17α-methylprogesterone and 19-norprogesterone, or of 17α-methyl-19-norprogesterone.[9][11][1] Related derivatives of 17α-methyl-19-norprogesterone include demegestone and trimegestone.[9][12][1]
History
Promegestone was first described in the literature in 1973 and was introduced for medical use in France in 1983.[9][10][11][5] It was developed by Roussel Uclaf in France.[5]
Society and culture
Generic names
Promegestone is the generic name of the drug and its INN, while promégestone is its DCF.[6][9][12] It is also known by its developmental code name R-5020 or RU-5020.[6][9][12]
Brand names
Promegestone is marketed exclusively under the brand name Surgestone.[6][12]
Availability
Promegestone is or has been marketed in France , Portugal, Tunisia, and Argentina .[6][12]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ 2.0 2.1 2.2 2.3 "Preclinical and clinical properties of trimegestone: a potent and selective progestin". Gynecological Endocrinology 23 (6): 310–319. June 2007. doi:10.1080/09513590701267727. PMID 17616854.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Pharmacology of progestogens". Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology 8 (Special Issue 1): 157–176. 2011. http://www.kup.at/kup/pdf/10168.pdf.
- ↑ 4.0 4.1 4.2 "[Promegestone, a new progestin]" (in fr). Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 12 (7): 697–710. 1983. PMID 6366037.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "To Market – 1983". Annual Reports in Medicinal Chemistry. 19. Academic Press. 11 September 1984. pp. 323–. ISBN 978-0-08-058363-1. https://books.google.com/books?id=oX-IT5QykwwC&pg=PA323.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "List of Progestins". https://www.drugs.com/international/promegestone.html.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 European Collaboration: Towards Drug Developement [sic] and Rational Drug Therapy: Proceedings of the Sixth Congress of the European Association for Clinical Pharmacology and Therapeutics Istanbul, June 24–28, 2003. Springer Science & Business Media. 6 December 2012. pp. 107–. ISBN 978-3-642-55454-4. https://books.google.com/books?id=Yk_xCAAAQBAJ&pg=PA107. "Investigation of the Pharmacokinetics and Metabolism of Promegestone in Healthy Female Volunteers Following Single Oral Administration of 1 mg Promegestone I Gualano V., 1Geneteau A., I Chassard D., I Fordham P., 2Schatz B. I Aster-Cephac, 3/5, Rue Eugene Millon, 75015 Paris, France 2Laboratoire Aventis, 46 Quai De La Rapee, F-75601 Paris Cedex 12, France. A single 1 mg oral dose of promegestone (Surgestonee, 2x0.5 mg) was given to 12 healthy premenopausal women. The aims were to determine the concentrations of promegestone and its metabolites and their pharmacokinet-ic parameters. Blood and urine samples were followed until 96 hours post dose. To avoid any interference with natural hormones, promegestone was given between day 7 and 10 of the menstrual cycle. Clinical safety and tolerability were good. Most of the minor adverse events observed were estimated possibly linked to the study drug (menstrual disorders) because classically related to progestins therapy. In addition, no clinically relevant biological modifications were observed. There was a stereoselective metabolism of promegestone in favor of the 21S hydroxy-promegestone, the main circulating compound in plasma (AUC ratio 5/R of about 21). Levels of 21S hydroxy-promegestone are about twice greater than that of unchanged promegestone. The plasma levels of the second metabolite, i.e. 21 R hydroxy-promegestone are far below these of either promegestone and 21S hydroxy-promegestone. Promegestone, 215 hydroxy- and 21R hydroxy-promegestone are not excreted in urine. About 3% of the dose was recov-ered in urine as sulfo and/or glucuro-conjugate 21S hydroxy-promegestone and about 1% of the dose as sulfo and/or glucuro conjugate 21R hydroxy-promegestone."
- ↑ 8.0 8.1 "The preclinical biology of a new potent and selective progestin: trimegestone". Steroids 68 (10–13): 915–920. November 2003. doi:10.1016/s0039-128x(03)00142-9. PMID 14667983.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 1026–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1026.
- ↑ 10.0 10.1 "Progesterone binding in the immature mouse and rat uterus". Steroids 22 (1): 89–98. July 1973. doi:10.1016/0039-128x(73)90073-1. PMID 4353432.
- ↑ 11.0 11.1 11.2 11.3 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 2935–36. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA2935-IA448.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 883–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA883.
- ↑ 13.0 13.1 "Stable and Specific Tracers". Reproductive Processes and Contraception. Biochemical Endocrinology. Springer. 1981. pp. 163–179. doi:10.1007/978-1-4684-3824-6_7. ISBN 978-1-4684-3826-0.
- ↑ "Double-blind trial of promegestone (R 5020) and lynestrenol in the treatment of benign breast disease". European Journal of Obstetrics, Gynecology, and Reproductive Biology 43 (3): 219–227. February 1992. doi:10.1016/0028-2243(92)90177-z. PMID 1563574.
- ↑ "Hormonal contraception in women at risk of vascular and metabolic disorders: guidelines of the French Society of Endocrinology". Annales d'Endocrinologie 73 (5): 469–487. November 2012. doi:10.1016/j.ando.2012.09.001. PMID 23078975.
- ↑ "The steroid promegestone is a noncompetitive antagonist of the Torpedo nicotinic acetylcholine receptor that interacts with the lipid-protein interface". Molecular Pharmacology 55 (2): 269–278. February 1999. doi:10.1124/mol.55.2.269. PMID 9927618.
- ↑ "The binding of a synthetic progestin, R5020 to transcortin and serum albumin". The Journal of Clinical Endocrinology and Metabolism 44 (5): 983–985. May 1977. doi:10.1210/jcem-44-5-983. PMID 858781.
- ↑ 18.0 18.1 Biochemical Actions of Hormones. Elsevier. 2 December 2012. pp. 314–. ISBN 978-0-323-15344-7. https://books.google.com/books?id=tX9GwWPsMbQC&pg=PA314.
- ↑ Progestogens in Obstetrics and Gynecology. Springer. 9 April 2015. pp. 34–. ISBN 978-3-319-14385-9. https://books.google.com/books?id=Ik8SCAAAQBAJ&pg=PA34.
Further reading
- "[Promegestone, a new progestin]" (in fr). Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 12 (7): 697–710. 1983. PMID 6366037.
- "[Clinical use of promegestone, a progestational agent with high specificity for receptors]" (in fr). Revue Francaise de Gynecologie et d'Obstetrique 79 (5): 423–426. May 1984. PMID 6396815.
 |