Chemistry:Tiletamine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV, IM, SC, Other |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| Chemical and physical data | |
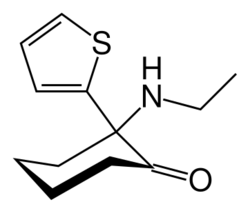
| Formula | C12H17NOS |
| Molar mass | 223.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist.[1] It is related chemically to ketamine.[2] Tiletamine hydrochloride exists as odorless white crystals.
It is used in veterinary medicine in the combination product Telazol (tiletamine/zolazepam, 50 mg/ml of each in 5 ml vial) as an injectable anesthetic for use in cats and dogs.[3][4][5] It is sometimes used in combination with xylazine (Rompun) to chemically immobilize large mammals such as polar bears[6] and wood bison.[7] Telazol is the only commercially available tiletamine product in the United States . It is contraindicated in patients of an ASA score of III or greater and in animals with CNS signs, hyperthyroidism, cardiac disease, pancreatic or renal disease, pregnancy, glaucoma, or penetrating eye injuries.[3]
Society and Culture
Recreational use of telazol has been documented.[8] Animal studies have also shown that tiletamine produces rewarding and reinforcing effects.[9] Products that combine Tiletamine and Zolazepam are classified as Schedule III controlled substances in the United States.[10] Otherwise, as noted by the DEA, tiletamine is unscheduled: “…[R]ules applicable to the scheduling of tiletamine and zolazepam as individual entities are not warranted [or in effect] at this time. Neither tiletamine nor zolazepam, as discrete substances, is perceived to pose a significant threat to the health and general welfare at this time…”[11]
References
- ↑ "Paradoxical convulsant action of a novel non-competitive N-methyl-D-aspartate (NMDA) antagonist, tiletamine". Brain Research 461 (2): 343–348. October 1988. doi:10.1016/0006-8993(88)90265-X. PMID 2846121.
- ↑ CID 26533 from PubChem
- ↑ 3.0 3.1 "Tiletamine". Drugs.com. https://www.drugs.com/international/tiletamine.html.
- ↑ "Telazol--a review of its pharmacology and use in veterinary medicine". Journal of Veterinary Pharmacology and Therapeutics 16 (4): 383–418. December 1993. doi:10.1111/j.1365-2885.1993.tb00206.x. PMID 8126757.
- ↑ "Tiletamine". Toxnet. U.S. National Library of Medicine. 21 January 2009. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+14176-49-9.
- ↑ "Anesthesia of polar bears using xylazine-zolazepam-tiletamine or zolazepam-tiletamine". Journal of Wildlife Diseases 39 (3): 655–664. July 2003. doi:10.7589/0090-3558-39.3.655. PMID 14567228.
- ↑ "Anesthesia of wood bison with medetomidine-zolazepam/tiletamine and xylazine-zolazepam/tiletamine combinations". The Canadian Veterinary Journal 41 (1): 49–53. January 2000. doi:10.4141/cjas61-007. PMID 10642872.
- ↑ "Abuse of telazol: an animal tranquilizer". Journal of Toxicology. Clinical Toxicology 39 (4): 399–402. 2001. doi:10.1081/clt-100105161. PMID 11527235.
- ↑ "Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, Zoletil®: difference between acute and repeated exposure". Behavioural Brain Research 233 (2): 434–442. August 2012. doi:10.1016/j.bbr.2012.05.038. PMID 22659394.
- ↑ "Lists of: Scheduling Actions, Controlled Substances, Regulated Chemicals". Drug Enforcement Administration. http://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf.
- ↑ "Schedules of Controlled Substances: Placement of Preparations Which Contain Both Tiletamine and Zolazepam into Schedule III". Drug Enforcement Administration. January 21, 1987. http://isomerdesign.com/Cdsa/FR/52FR2221.pdf.
External links
 |

