Chemistry:Cartazolate
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
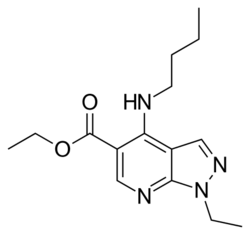
| Formula | C15H22N4O2 |
| Molar mass | 290.367 g·mol−1 |
| 3D model (JSmol) | |
| |
Cartazolate (SQ-65,396) is a drug of the pyrazolopyridine class. It acts as a GABAA receptor positive allosteric modulator at the barbiturate binding site of the complex and has anxiolytic effects in animals.[1][2][3][4] It is also known to act as an adenosine antagonist at the A1 and A2 subtypes and as a phosphodiesterase inhibitor.[5][6] Cartazolate was tested in human clinical trials and was found to be efficacious for anxiety but was never marketed.[7] It was developed by a team at E.R. Squibb and Sons in the 1970s.[8]
See also
References
- ↑ "In vitro modulation by SQ 20009 and SQ 65396 of GABA receptor binding in rat CNS membranes". European Journal of Pharmacology 62 (2–3): 225–8. March 1980. doi:10.1016/0014-2999(80)90281-2. PMID 6103810.
- ↑ "Action of pyrazolopyridines as modulators of [3H]flunitrazepam binding to the gaba/benzodiazepine receptor complex of the cerebellum". European Journal of Pharmacology 70 (2): 183–93. March 1981. doi:10.1016/0014-2999(81)90213-2. PMID 6114867.
- ↑ "Perturbation of benzodiazepine receptor binding by pyrazolopyridines involves picrotoxinin/barbiturate receptor sites". Journal of Neuroscience 1 (5): 471–7. May 1981. doi:10.1523/JNEUROSCI.01-05-00471.1981. PMID 7050308.
- ↑ "Biochemical characterization of an isolated and functionally reconstituted gamma-aminobutyric acid/benzodiazepine receptor". Journal of Neurochemistry 54 (3): 751–61. March 1990. doi:10.1111/j.1471-4159.1990.tb02315.x. PMID 2154549.
- ↑ "Non-xanthine heterocycles: activity as antagonists of A1- and A2-adenosine receptors". Biochemical Pharmacology 37 (4): 655–64. February 1988. doi:10.1016/0006-2952(88)90139-6. PMID 2829919.
- ↑ Wachtel H (1982). "Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3', 5'-monophosphate phosphodiesterase inhibitors". Psychopharmacology 77 (4): 309–16. doi:10.1007/BF00432761. PMID 6182575.
- ↑ O'Brien, Robert (1986). Receptor binding in drug research. New York: Dekker. p. 519. ISBN 0-8247-7548-1. https://books.google.com/books?id=GDE2VTeeHPIC&q=cartazolate&pg=PA78.
- ↑ Hoehn, Hans & Theodor Denzel, "Amino derivatives of pyrazolopyridine carboxamides", US patent 3966746, published 1976-06-29
 |

