Chemistry:Testosterone propionate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Testoviron, others |
| Other names | TP; Testosterone propanoate; Testosterone 17β-propanoate; Propionyltestosterone; NSC-9166 |
| Routes of administration | Intramuscular injection, buccal |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: very low Intramuscular: very high |
| Metabolism | Liver |
| Elimination half-life | Intramuscular: 0.8 days (~20 hours)[1][2][3] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H32O3 |
| Molar mass | 344.495 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Testosterone propionate, sold under the brand name Testoviron among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels in men.[4][1][5] It has also been used to treat breast cancer in women.[6] It is given by injection into muscle usually once every two to three days.[5][7][8]
Side effects of testosterone propionate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[5] Testosterone supplementation is also known to reduce the threshold for aggressive behavior in men.[9] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[10][5] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy.[5] Testosterone propionate is a testosterone ester and a relatively short-acting prodrug of testosterone in the body.[7][4][1] Because of this, it is considered to be a natural and bioidentical form of testosterone.[11]
Testosterone propionate was discovered in 1936 and was introduced for medical use in 1937.[12][4] It was the first testosterone ester to be marketed, and was the major form of testosterone used in medicine until about 1960.[4][5] The introduction of longer-acting testosterone esters like testosterone enanthate, testosterone cypionate, and testosterone undecanoate starting in the 1950s resulted in testosterone propionate mostly being superseded.[4][5] As such, it is rarely used today.[5][13] In addition to its medical use, testosterone propionate is used to improve physique and performance.[5] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[5]
Medical uses
Testosterone propionate is used primarily in androgen replacement therapy. It is specifically approved for the treatment of hypogonadism in men, breast cancer, low sexual desire, delayed puberty in boys, and menopausal symptoms.[14]
| Route | Medication | Form | Dosage | |
|---|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day | |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | ||
| Calusterone | Tablet | 40–80 mg 4x/day | ||
| Normethandrone | Tablet | 40 mg/day | ||
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day | |
| Injection (IM or SC) | Testosterone propionate | Oil solution | 50–100 mg 3x/week | |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | ||
| Methandriol | Aqueous suspension | 100 mg 3x/week | ||
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | ||
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | ||
| Metenolone enanthate | Oil solution | 400 mg 3x/week | ||
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | ||
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | ||||
Available forms
Testosterone propionate is usually provided as an oil solution for use by intramuscular injection.[5] It was also previously available as an 30 mg or 50 mg aqueous suspension.[15] Buccal tablets of testosterone propionate were previously available as well.[5]
Side effects
Side effects of testosterone propionate include virilization among others.[5]
Testosterone propionate is often a painful injection, which is attributed to its short ester chain.[5]
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Testosterone propionate is a prodrug of testosterone and is an androgen and anabolic–androgenic steroid (AAS). That is, it is an agonist of the androgen receptor (AR).
Pharmacokinetics
Testosterone propionate is administered in oil via intramuscular injection.[1][2] It has a relatively short elimination half-life and mean residence time of 2 days and 4 days, respectively.[1][2] As such, it has a short duration of action and must be administered two to three times per week.[16]
Intramuscular injection of testosterone propionate as an oil solution, aqueous suspension, and emulsion has been compared.[17]
| Testosterone ester | Form | Route of administration | Elimination half-life | Mean residence time |
|---|---|---|---|---|
| Testosterone undecanoate | Oil-filled capsules | Oral | 1.6 hours | 3.7 hours |
| Testosterone propionate | Oil solution | Intramuscular injection | 0.8 days | 1.5 days |
| Testosterone enanthate | Castor oil solution | Intramuscular injection | 4.5 days | 8.5 days |
| Testosterone undecanoate | Tea seed oil solution | Intramuscular injection | 20.9 days | 34.9 days |
| Testosterone undecanoate | Castor oil solution | Intramuscular injection | 33.9 days | 36.0 days |
| Testosterone buciclatea | Aqueous suspension | Intramuscular injection | 29.5 days | 60.0 days |
| Notes: Testosterone cypionate has very similar pharmacokinetics to TE. Footnotes: a = Never marketed. Sources: See template. | ||||
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–20 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–20 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
Chemistry
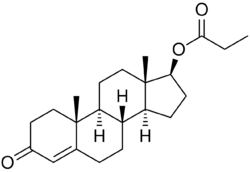
Testosterone propionate, or testosterone 17β-propanoate, is a synthetic androstane steroid and a derivative of testosterone.[18][19] It is an androgen ester; specifically, it is the C17β propionate (propanoate) ester of testosterone.[18][19]
History
Testosterone esters were synthesized for the first time in 1936, and were found to have greatly improved potency relative to testosterone.[12] Among the esters synthesized, testosterone propionate was the most potent, and for this reason, was selected for further development, subsequently being marketed.[12] Testosterone propionate was introduced in 1937 by Schering AG in Germany under the brand name Testoviron.[5] It was the first commercially available form of testosterone, and the first testosterone ester, to be introduced.[4][20] The medication was the major form of testosterone used medically before 1960.[5] Buccal testosterone propionate tablets were introduced for medical use in the mid-to-late 1940s under the brand name Oreton Buccal Tablets.[21][22][23] An aqueous suspension of testosterone propionate was marketed by Ciba by 1950.[24] In the 1950s, longer-acting testosterone esters like testosterone enanthate and testosterone cypionate were introduced and superseded testosterone propionate.[4] Although rarely used nowadays due to its short duration,[13] testosterone propionate remains medically available.[5]
Society and culture
Generic names
Testosterone propionate is the generic name of the drug and its USAN and BAN.[18][19][25][26] It has also been referred to as testosterone propanoate or as propionyltestosterone.[18][19][25][26]
Brand names
Testosterone propionate is or has been marketed under a variety of brand names, including, among numerous others:[18][19][25][26]
- Agrovirin
- Andronate
- Andrusol-P
- Anertan[15]
- Masenate
- Neo-Hombreol
- Oreton
- Perandren
- Synandrol
- Testoviron
Availability
Testosterone propionate is no longer available commercially in the United States except via a compounding pharmacy.[27]
Legal status
Testosterone propionate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[28][29]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Testosterone Therapy". Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. 13 January 2010. pp. 441–446. ISBN 978-3-540-78355-8. https://books.google.com/books?id=mEgckDNkonUC&pg=PA441.
- ↑ 2.0 2.1 2.2 "Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies". European Journal of Endocrinology 140 (5): 414–419. May 1999. doi:10.1530/eje.0.1400414. PMID 10229906.
- ↑ "Testosterone Replacement Therapy". Sexual Medicine. Springer. 2019. pp. 79–93. doi:10.1007/978-981-13-1226-7_8. ISBN 978-981-13-1225-0.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "Testosterone preparations for clinical use in males". Testosterone: Action, Deficiency, Substitution. Cambridge University Press. 26 July 2012. pp. 9,315–. ISBN 978-1-107-01290-5. https://books.google.com/books?id=MkrAPaQ4wJkC&pg=PA315.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 Anabolics. Molecular Nutrition Llc. 2011. pp. 357–361,413,426,607,677. ISBN 978-0-9828280-1-4. https://books.google.com/books?id=afKLA-6wW0oC&pg=PT357.
- ↑ "Testosterone therapy in women: a review". International Journal of Impotence Research 17 (5): 399–408. 2005. doi:10.1038/sj.ijir.3901334. PMID 15889125.
- ↑ 7.0 7.1 Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 1185,1187. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1185.
- ↑ The Leydig Cell in Health and Disease. Springer Science & Business Media. 28 October 2007. pp. 423–. ISBN 978-1-59745-453-7. https://books.google.com/books?id=x4ttqKIAOg0C&pg=PA423.
- ↑ "Is testosterone linked to human aggression? A meta-analytic examination of the relationship between baseline, dynamic, and manipulated testosterone on human aggression". Hormones and Behavior 123: 104644. July 2020. doi:10.1016/j.yhbeh.2019.104644. PMID 31785281. http://clok.uclan.ac.uk/33858/1/33858Geniole%20et%20al%20Revised%20Oct%2030%202019.pdf.
- ↑ "Pharmacology of anabolic steroids". British Journal of Pharmacology 154 (3): 502–521. June 2008. doi:10.1038/bjp.2008.165. PMID 18500378.
- ↑ "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". The Journal of Clinical Endocrinology and Metabolism 101 (4): 1318–1343. April 2016. doi:10.1210/jc.2016-1271. PMID 27032319.
- ↑ 12.0 12.1 12.2 "The prolonged treatment of castrated and ovariectomized rats with testosterone propionate". The Biochemical Journal 31 (3): 475–485. March 1937. doi:10.1042/bj0310475. PMID 16746360.
- ↑ 13.0 13.1 Practical Urology: Essential Principles and Practice: Essential Principles and Practice. Springer Science & Business Media. 10 May 2011. pp. 228–. ISBN 978-1-84882-034-0. https://books.google.com/books?id=A9m8TkdCUqEC&pg=PA228.
- ↑ "Testosterone propionate". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800013172.
- ↑ 15.0 15.1 Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. 8 March 2013. pp. 21–. ISBN 978-3-7091-5694-0. https://books.google.com/books?id=Hte1BgAAQBAJ&pg=PA21.
- ↑ "Hypogonadism and Hormone Replacement in Men with Cancers". Medical Care of Cancer Patients. PMPH-USA. 2009. pp. 247–. ISBN 978-1-60795-008-0. https://books.google.com/books?id=XxfjqF1A0TkC&pg=PA247.
- ↑ "17-Ketosteroid Excretion and Modes of Administering Testosterone Preparations". Ciba Foundation Symposium - Steroid Hormone Administration (Book II of Colloquia on Endocrinology, Vol. 3). Novartis Foundation Symposia. John Wiley & Sons. 1952. pp. 304–322. doi:10.1002/9780470715154.ch7. ISBN 9780470715154.
- ↑ 18.0 18.1 18.2 18.3 18.4 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 641–642. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA641.
- ↑ 19.0 19.1 19.2 19.3 19.4 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1002–1004. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA1002.
- ↑ "Newer hormonal preparations". California Medicine 92 (2): 121–124. February 1960. PMID 13849734.
- ↑ The Mississippi Doctor. 1946. p. 7. https://books.google.com/books?id=YvkfAQAAMAAJ.
- ↑ The Midwestern Druggist .... 1948. p. 28. https://books.google.com/books?id=K-IrAQAAMAAJ.
- ↑ "New Prescription Products". Journal of the American Pharmaceutical Association (Practical Pharmacy Ed.) 10 (4): 198–206. 1949. doi:10.1016/S0095-9561(16)31795-9. ISSN 0095-9561.
- ↑ "Employment of androgens in gynecology". Acta Obstetricia et Gynecologica Scandinavica 30 (1): 106–127. 1950. doi:10.3109/00016345009154942. PMID 14777285.
- ↑ 25.0 25.1 25.2 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA270.
- ↑ 26.0 26.1 26.2 "Testosterone". Drugs.com. https://www.drugs.com/international/testosterone.html.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/daf/.
- ↑ "Criminalistics: Introduction to Controlled Substances". Drug Abuse Handbook (Second ed.). CRC Press. 21 December 2006. pp. 30–. ISBN 978-1-4200-0346-8. https://books.google.com/books?id=ZjrMBQAAQBAJ&pg=PA30.
- ↑ Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. 5 August 2016. pp. 50–. ISBN 978-1-77172-066-3. https://books.google.com/books?id=dNgoDwAAQBAJ&pg=PA50.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |

