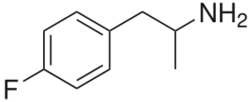
Chemistry:4-Fluoroamphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C9H12FN |
| Molar mass | 153.200 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
4-Fluoroamphetamine (4-FA; 4-FMP; PAL-303; "Flux"), also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.[2][3]
Usage
4-FA is popular in the Netherlands where it is predominantly used for its specific effects (77% of users) rather than its legal status (18%).[4] 4-FA has become illegal since May 2017.[5]
Effects
The subjective effects of 4-fluoroamphetamine include euphoria which some find similar to the effects of MDMA and amphetamine,[4] increased energy (stimulation), mood elevation, feelings of warmth and empathy, excessive talking, bruxism, and suppressed appetite (anorexic). The general course of effects involves primarily empathogenic effects for the first few hours, which fades out as increased stimulation develops over the next several hours.
The dopamine reuptake inhibition produced by 4-FA is stronger than that of either 4-CA or 4-IA.[6] 4-FA also produces less hyperthermia than similar compounds such as PMA, 3-MTA and 4-methylamphetamine.
Common acute side effects are nausea, headaches, increased heart rate and insomnia.
Chemistry
4-FA reacts with reagent testing to give a semi-unique array of colors which can be used to aid its identification.
| Reagent | Reaction color |
|---|---|
| Marquis | No reaction[7] |
| Mandelin | Pale Blue[7][8] |
| Liebermann | Orange[7][8] |
| Froehde | Faint purple/brown[7] or no reaction. |
Pharmacology
4-Fluoroamphetamine is a releasing agent and reuptake inhibitor of dopamine, serotonin, and norepinephrine.[9] The respective EC50 values are 2.0 x 10−7 M, 7.3 x 10−7 M, and 0.37 x 10−7 M, while the IC50 values are 7.7 x 10−7 M, 68 x 10−7 M, and 4.2 x 10−7 M.[3]
Regarding the metabolic fate of 4-FA, the C-F bond at the 4-position on the phenyl ring likely resists deactivation in the liver by cytochrome P450 oxidase.[10] [11]
Neurotoxicity
4-FA does not cause long-lasting depletion of brain serotonin, unlike its analogs 4-CA and 4-BA.[12] This is thought to "reflect the inability of the fluoro-compound to be metabolized in the same way as the other haloamphetamines."[13]
Neurotoxicity does not increase down the series of para-halogenated amphetamine derivatives, even though serotonin releasing potency does follow this trend. For example, 4-iodoamphetamine is less toxic than is 4-chloroamphetamine.[6][14] Hence, this property is not related to serotonin releasing potency as such, since PAL-287 was reported to be not at all neurotoxic even though it is a powerful 5-HT releasing agent.[15] It is unclear where 4-methylamphetamine fits in on the neurotoxicity scale. The extensive serotonergic neurotoxicity of 4-chloroamphetamine (and its brominated derivative), and the increased serotonergic toxicity of 4-methylamphetamine[16] suggest that para-substitution seems to increase overall serotonergic (neuro)toxicity, compared to amphetamine. Exceptions include 4-MTA, a para-substituted, non-neurotoxic amphetamine.[17][18][19]
Toxicology
The LD50 (mouse; i.p.) of 4-FA is 46 mg/kg.[20]
Fluoroamphetamine (isomer not determined) in a capsule mixed with 25C-NBOMe was associated with three deaths in Melbourne in 2017.[21]
Legal status
As of October 2015, 4-FA is a controlled substance in China.[22] 4-FA is banned in the Czech Republic.[23] As of 25 May 2017 4-FA is a controlled substance in the Netherlands.[24] 4-FA is also controlled in Australia, Belgium, UK, Germany, Israel, Slovakia, Bulgaria, Chile, Brazil, Canada, Croatia, Sweden, New Zealand and France.[citation needed]
See also
- 2-Fluoroamphetamine (2-FA)
- 3-Fluoroamphetamine (3-FA)
- 3,4-Difluoroamphetamine
- 4-Fluoromethamphetamine (4-FMA)
- 4-Fluoromethcathinone (4-FMC)
- 4'-Fluoro-4-methylaminorex
- para-Bromoamphetamine (PBA)
References
- ↑ "Substance Details 4-Fluoroamphetamine". https://www.unodc.org/LSS/Substance/Details/35999356-e8a6-428b-a537-90e09d258e0c.
- ↑ "Isomeric fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs)". Forensic Science International 148 (2–3): 143–156. March 2005. doi:10.1016/j.forsciint.2004.05.003. PMID 15639609.
- ↑ 3.0 3.1 "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–137. March 2007. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ 4.0 4.1 "4-Fluoroamphetamine in the Netherlands: more than a one-night stand". Addiction 110 (7): 1138–1143. July 2015. doi:10.1111/add.12932. PMID 25808511.
- ↑ "Het is nu officieel: de partydrug 4-FA is verboden". 25 May 2017. https://nos.nl/op3/artikel/2174904-het-is-nu-officieel-de-partydrug-4-fa-is-verboden.html.
- ↑ 6.0 6.1 "Psychostimulant-like effects of p-fluoroamphetamine in the rat". European Journal of Pharmacology 287 (2): 105–113. December 1995. doi:10.1016/0014-2999(95)00478-5. PMID 8749023.
- ↑ 7.0 7.1 7.2 7.3 "4-FA reaction colour results with liebermann and froehde reagent test kits". Reagent Tests UK. 3 January 2016. https://www.reagent-tests.uk/blog/4-fa-reaction-colour-results-with-liebermann-and-froehde-reagent-test-kits/.
- ↑ 8.0 8.1 "[Analytical data of designated substances (Shitei-Yakubutsu) controlled by the Pharmaceutical Affairs Law in Japan, part II: Color test and TLC"]. Yakugaku Zasshi 128 (6): 981–987. June 2008. doi:10.1248/yakushi.128.981. PMID 18520145. https://www.jstage.jst.go.jp/article/yakushi/128/6/128_6_981/_pdf.
- ↑ "Pharmacokinetic properties of 4-fluoroamphetamine in serum and oral fluid after oral ingestion". Drug Testing and Analysis 11 (7): 1028–1034. July 2019. doi:10.1002/dta.2595. PMID 30912312.
- ↑ "The complexities inherent in attempts to decrease drug clearance by blocking sites of CYP-mediated metabolism". Current Opinion in Drug Discovery & Development 9 (1): 101–109. January 2006. PMID 16445122.
- ↑ "Pharmacokinetic properties of 4-fluoroamphetamine in serum and oral fluid after oral ingestion". Drug Testing and Analysis 11 (7): 1028–1034. July 2019. doi:10.1002/dta.2595. PMID 30912312.
- ↑ "Neurotoxic action of halogenated amphetamines". Annals of the New York Academy of Sciences 305 (1): 289–304. June 1978. doi:10.1111/j.1749-6632.1978.tb31530.x. PMID 81648. Bibcode: 1978NYASA.305..289H.
- ↑ "Comparison of 4-chloro-, 4-bromo- and 4-fluoroamphetamine in rats: drug levels in brain and effects on brain serotonin metabolism". Neuropharmacology 14 (10): 739–746. October 1975. doi:10.1016/0028-3908(75)90099-4. PMID 1196472.
- ↑ "5-Iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine". Pharmacology, Biochemistry, and Behavior 38 (1): 135–139. January 1991. doi:10.1016/0091-3057(91)90601-W. PMID 1826785.
- ↑ "Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration". The Journal of Pharmacology and Experimental Therapeutics 313 (3): 1361–1369. June 2005. doi:10.1124/jpet.104.082503. PMID 15761112.
- ↑ "4-Methyl-amphetamine: a health threat for recreational amphetamine users". Journal of Psychopharmacology 27 (9): 817–822. September 2013. doi:10.1177/0269881113487950. PMID 23784740.
- ↑ "p-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent". European Journal of Pharmacology 229 (1): 31–38. December 1992. doi:10.1016/0014-2999(92)90282-9. PMID 1473561.
- ↑ "Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)". The Journal of Pharmacology and Experimental Therapeutics 279 (3): 1261–1267. December 1996. PMID 8968349.
- ↑ "In vitro neuronal and vascular responses to 5-hydroxytryptamine: modulation by 4-methylthioamphetamine, 4-methylthiomethamphetamine and 3,4-methylenedioxymethamphetamine". European Journal of Pharmacology 444 (1–2): 61–67. May 2002. doi:10.1016/S0014-2999(02)01586-8. PMID 12191583.
- ↑ Amphetamines and Related Compounds. New York: Raven Press. 1970. p. 28.
- ↑ "News: March 2017 – Australia: "Ecstasy" capsules containing NPS are related to several deaths and severe intoxications in Melbourne". https://www.unodc.org/LSS/announcement/Details/48940b13-df58-4da2-a66b-d54a8f7362f7.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (in cs). Ministerstvo zdravotnictví. http://www.mzcr.cz/Admin/_upload/files/3/Nov%C3%A9%20PL.pdf.
- ↑ "Vanaf vandaag is partydrug 4-FA officieel verboden - maar of dat helpt?" (in nl). de Volkskrant. 25 May 2017. http://www.volkskrant.nl/binnenland/vanaf-vandaag-is-partydrug-4-fa-officieel-verboden-maar-of-dat-helpt~a4496813/.
External links
 |


