Chemistry:Parahexyl
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H32O2 |
| Molar mass | 328.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
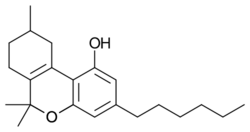
Parahexyl (Synhexyl, n-hexyl-Δ3-THC, (C6)-Δ6a(10a)-THC) is a synthetic homologue of THC which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis.[1][2][3]
Parahexyl is similar in both structure and activity to THC, differing only in the position of one double bond and the lengthening of the 3-pentyl chain by one CH2 group to n-hexyl.[4] Parahexyl produces effects typical of other cannabinoid receptor agonists in animals. It has a somewhat higher oral bioavailability than THC itself but is otherwise very similar.[5] Presumably, it acts as a CB1 agonist in the same way as THC, but as there has been no research published using parahexyl since the discovery of the CB1 receptor, this has not been definitively confirmed.
Parahexyl was occasionally used as an anxiolytic in the mid-20th century, the dosage ranging from 5 mg to 90 mg.[6]
Parahexyl was made illegal under United Nations convention in 1982 on the basis of its structural similarity and similar effects profile to THC. Parahexyl was placed into the most restrictive Schedule 1 as a compound with no medical use, despite the now-known medical uses for cannabinoids.
Isomerism
At least three isomers of parahexyl have been studied and are known to be active as cannabinoids. Parahexyl itself (i.e. the Δ6a(10a) isomer) has not had any significant use in scientific research since it was banned internationally in the early 1980s; however, the Δ8 and Δ9 isomers are both known to be cannabinoid receptor agonists, and Δ8-parahexyl has the code number JWH-124,[7][8] while Δ9-parahexyl has been isolated from Cannabis plant material and assigned the name tetrahydrocannabihexol.[9]
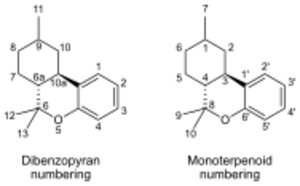
| 7 double bond isomers of parahexyl and their 30 stereoisomers | |||||||
|---|---|---|---|---|---|---|---|
| Dibenzopyran numbering | Monoterpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | |||
| Δ6a(7)-parahexyl | 9 and 10a | 3-hexyl-8,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ4-parahexyl | 1 and 3 | 4 | No | unscheduled |
| Δ7-parahexyl | 6a, 9 and 10a | 3-hexyl-6a,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ5-parahexyl | 1, 3 and 4 | 8 | No | unscheduled |
| Δ8-parahexyl | 6a and 10a | 3-hexyl-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ6-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ9,11-parahexyl | 6a and 10a | 3-hexyl-6a,7,8,9,10,10a-hexahydro-6,6-dimethyl-9-methylene-6H-dibenzo[b,d]pyran-1-ol | Δ1(7)-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ9-parahexyl | 6a and 10a | 3-hexyl-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ1-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ10-parahexyl | 6a and 9 | 3-hexyl-6a,7,8,9-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ2-parahexyl | 1 and 4 | 4 | No | unscheduled |
| Δ6a(10a)-parahexyl | 9 | 3-hexyl-7,8,9,10-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ3-parahexyl | 1 | 2 | No | Schedule I |
Note that 6H-dibenzo[b,d]pyran-1-ol is the same as 6H-benzo[c]chromen-1-ol.
See also
- Delta-3-Tetrahydrocannabinol
- Hexahydrocannabihexol
- Tetrahydrocannabutol
- Tetrahydrocannabiphorol
- Tetrahydrocannabivarin
References
- ↑ Adams R, et al. Tetrahydrocannabinol Homologs with Marihuana Activity. IX. J. Am. Chem. Soc. 1941; 63(7):1971–1973. doi:10.1021/ja01852a052
- ↑ "New Analogs of Tetrahydrocannabinol. XIX". J. Am. Chem. Soc. 71 (5): 1624–1628. 1949. doi:10.1021/ja01173a023.
- ↑ Ask Dr. Shulgin Online March 7, 2001
- ↑ "[Studies on hallucinogens. VII Synthesis of parahexyl]" (in ja). Eisei Shikenjo Hokoku. Bulletin of National Institute of Hygienic Sciences 49 (92): 46–50. 1974. PMID 4477495.
- ↑ "EEG effects of hallucinogens and cannabinoids using sleep-waking behavior as baseline". Pharmacology, Biochemistry, and Behavior 12 (1): 99–105. January 1980. doi:10.1016/0091-3057(80)90422-0. PMID 6102770.
- ↑ Farmakologia. Warsaw: PZWL. 1950. p. 89.
- ↑ "Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists". The Journal of Pharmacology and Experimental Therapeutics 290 (3): 1065–79. September 1999. PMID 10454479.
- ↑ "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry 8: 17–39. 2016. doi:10.4137/PMC.S32171. PMID 27398024.
- ↑ "Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: cannabidihexol". Scientific Reports 10 (1): 22019. December 2020. doi:10.1038/s41598-020-79042-2. PMID 33328530. Bibcode: 2020NatSR..1022019L.
 |