Chemistry:2-Fluorodeschloroketamine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H16FNO |
| Molar mass | 221.275 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
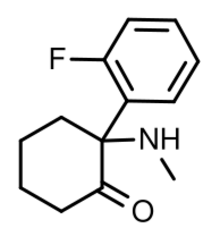
2-Fluorodeschloroketamine (also known as 2'-Fl-2-Oxo-PCM, Fluoroketamine and 2-FDCK) is a dissociative anesthetic[1] related to ketamine. Its sale and use as a designer drug has been reported in various countries.[2][3][4] It is an analogue of ketamine where the chlorine group has been replaced by fluorine. Due to its recent emergence, the pharmocological specifics of the compound are mostly unclear.
History
The synthesis of 2-FDCK was first described in a 2013 paper as part of a larger effort to synthesize and evaluate new anesthetic drugs based on ketamine and its analogues.[1] Ketamine itself was first introduced in 1964 and was approved for clinical use in 1970. Since then it has become one of the most important and applicable general anesthetics as well as a popular recreational drug.
The use of 2-FDCK as a designer drug has been reported in various countries.[2][5][6] Many of these new psychoactive substances (NPS) appear on the drug market in order to circumvent existing drug policies. 2-FDCK was first formally notified by the EMCDDA in 2016, alongside 65 other new substances.[6] Due to its recent appearance, little research has been done on the compound so far.
Chemistry
Structure
The full chemical name of 2-FDCK is 2-(2-fluorophenyl)-2-(methylamino)cyclohexan-1-one.
2-FDCK belongs to a class of compounds called arylcyclohexylamines which contains various other drugs such as PCP and ketamine. Their general structure consists of a cyclohexylamine unit with an aryl group attached to the same carbon as the amine. 2-FDCK has an o-fluorophenyl group as an aryl substituent and the amine group is methylated. The cyclohexyl ring features a ketone group next to the amine position.
The chemical structure of 2-FDCK differs from ketamine only in that there is a fluorine atom attached to the phenyl group. Ketamine has a chlorine atom in that position.[7]
Synthesis
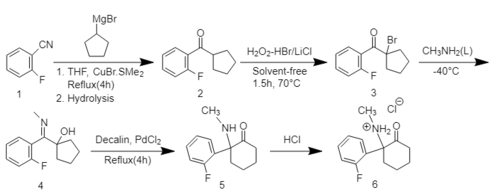
2-FDCK can be synthesized in a five-step reaction process.[1] First 2-fluorobenzonitrile reacts with the Grignard reagent cyclopentyl magnesium bromide followed by a bromination reaction to obtain α-bromocyclopentyl-(2-fluorophenyl)-ketone. The reaction of the obtained ketone with methylamine at -40 °C then results in the formation of α-hydroxycyclopentyl-(2-fluorophenyl)-N-methylamine. Finally, the five-membered ring cyclopentanol form is expanded to a cyclohexylketone form by a thermal rearrangement reaction. HCl is used to create a water-soluble HCl salt of 2-FDCK.
Detection
2-FDCK and its metabolites can be detected in urine with the use of liquid chromatography mass spectrometry (LC/MS).[4]
Pharmacology
Metabolism

The metabolism of 2-FDCK is analogous to that of ketamine: the enzymes CYP2B6 and CYP3A4, the latter to a lesser extent, metabolise 2-FDCK to Nor-2FDCK via N-demethylation. This is further metabolised either to dehydronor-2FDCK by CYP2B6 or to hydroxynor-2FDCK by CYP2A6 and CYP2B6.[3]
In general, the 2-FDCK equivalent shows stronger docking to CYP2B6 in simulations, as well as slower metabolism rate, than the more well-known Ketamine. The lipophilicity is observed to be lower for 2-FDCK than for Ketamine.[3] In vitro to in vivo extrapolation (IVIVE) predicts that in the body, 2-FDCK shows a lower intrinsic hepatic clearance than ketamine. Both of these characteristics would suggest that the effects of 2-FDCK last longer than those of ketamine.[2]
Pharmacodynamics
2-FDCK is structurally similar to ketamine, so a similar mechanism of action is expected,[8] but there has been no study done to confirm this. Due to the halogen in the 2 position not being a chlorine but a fluorine, the molecule is less polar.[3] This could influence binding to proteins, such as the NMDA receptor that ketamine primarily binds to and acts as an antagonist towards.
Comparison to other halogen-substituted ketamine variants
For general (halogen) substitutions of ketamine, docking strength for CYP2B6 follows the pattern H < Br < Cl < F. The parameter of internal clearance follows the pattern Br > Cl > F > H. Lastly, Km (Michaelis constant) follows the pattern of Br < Cl < F < H, and as such the in-vitro metabolism rate follows the inverse pattern, namely Br > Cl > F > H.[4]
Effects
Dosage
2-FDCK is very new and not yet sufficiently studied. Therefore information on dosage can only be obtained from purely anecdotal sources.[9]
Possible effects and dangers
In 2019, 2-FDCK was found in poisoned individuals in Hong Kong in combination with other ketamine-type drugs.[4] 2-FDCK was not assumed to be an impurity because it was present in high levels while ketamine was present at trace levels. The actual drug specimens were not available, so it was not possible to verify this. Clinical effects were observed but because the drug was taken in combination with other ones, it was not possible to determine what effects were caused by which drug. The clinically observed effects of the patients were: impaired consciousness, agitation, abnormal behaviour, hypertension and tachycardia. Most patients were discharged from the hospital after a few days of supportive treatment.
Legal status
Due to the fast emergence of NPS, new substances such as 2-FDCK are often not yet specifically mentioned in controlled substance legislation. As a result, NPS are sometimes marketed as 'legal highs'. 2-FDCK is currently illegal in Italy[10] Japan,[11] Latvia,[12] Singapore,[13] Sweden,[14] Switzerland,[15] as well as being covered by blanket bans in Canada,[16] Belgium,[17] and the UK.[18]
See also
- 3-Fluorodeschloroketamine
- Bromoketamine
- Deschloroketamine
- Fluorexetamine
- Methoxyketamine
- Trifluoromethyldeschloroketamine
References
- ↑ 1.0 1.1 1.2 1.3 "Synthesis of 2-(2-Fluorophenyl)-2-methylamino-Cyclohexanone as a New Ketamine Derivative" (in en). Synthetic Communications 44 (14): 2021–2028. 2014-07-18. doi:10.1080/00397911.2014.885053. https://figshare.com/articles/journal_contribution/Synthesis_of_2_2_Fluorophenyl_2_methylamino_Cyclohexanone_as_a_New_Ketamine_Derivative/1003833.
- ↑ 2.0 2.1 2.2 "Ketamine analogues: Comparative toxicokinetic in vitro-in vivo extrapolation and quantification of 2-fluorodeschloroketamine in forensic blood and hair samples". Journal of Pharmaceutical and Biomedical Analysis 180: 113049. February 2020. doi:10.1016/j.jpba.2019.113049. PMID 31881397.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Halogen Substitution Influences Ketamine Metabolism by Cytochrome P450 2B6: In Vitro and Computational Approaches". Molecular Pharmaceutics 16 (2): 898–906. February 2019. doi:10.1021/acs.molpharmaceut.8b01214. PMID 30589555.
- ↑ 4.0 4.1 4.2 4.3 "Emergence of new psychoactive substance 2-fluorodeschloroketamine: Toxicology and urinary analysis in a cluster of patients exposed to ketamine and multiple analogues". Forensic Science International 312: 110327. July 2020. doi:10.1016/j.forsciint.2020.110327. PMID 32460225.
- ↑ "Ketamine analogues multiplying in Hong Kong". Hong Kong Medical Journal = Xianggang Yi Xue Za Zhi 25 (2): 169. April 2019. doi:10.12809/hkmj197863. PMID 30971512.
- ↑ 6.0 6.1 European Monitoring Centre for Drugs and Drug Addiction (2017), EMCDDA–Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA, Implementation reports, Publications Office of the European Union, Luxembourg.
- ↑ "Compound Summary for CID 13771618, Fluoroketamine". PubChem. National Center for Biotechnology Information (2021).. https://pubchem.ncbi.nlm.nih.gov/compound/Fluoroketamine.
- ↑ "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis 6 (7–8): 614–32. 2014. doi:10.1002/dta.1620. PMID 24678061.
- ↑ "2-FDCK". PsychonautWiki. https://psychonautwiki.org/wiki/2-Fluorodeschloroketamine.
- ↑ "Article 1" (in Italian). Aggiornamento delle tabelle contenenti l'indicazione delle sostanze stupefacenti e psicotrope, di cui al decreto del Presidente della Repubblica 9 ottobre 1990, n. 309 e successive modificazioni ed integrazioni. Inserimento nella tabella I e nella tabella IV di nuove sostanze psicoattive. 13 March 2020. https://www.gazzettaufficiale.it/eli/id/2020/03/30/20A01820/sg.
- ↑ "指定薬物一覧" (PDF) (in Japanese). Ministry of Health, Labour and Welfare
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" (in Latvian). Latvijas Republikas tiesību akti. https://likumi.lv/doc.php?id=121086.
- ↑ "Misuse of Drugs Act – Singapore Statutes Online". sso.agc.gov.sg. https://sso.agc.gov.sg/Act/MDA1973.
- ↑ "Förordning (1999:58) om förbud mot vissa hälsofarliga varor Svensk författningssamling 1999:1999:58 t.o.m. SFS 2019:631 – Riksdagen" (in Swedish). Riksdagsförvaltningen. www.riksdagen.se. https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/forordning-199958-om-forbud-mot-vissa_sfs-1999-58.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Der Bundesrat. https://www.fedlex.admin.ch/eli/cc/2011/363/de.
- ↑ "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". https://laws-lois.justice.gc.ca/eng/acts/C-38.8/.
- ↑ Dossier Nieuwe Psychoactieve Stoffen. Brussel: VAD. 2019. https://www.vad.be/assets/dossier-nieuwe-psychoactieve-stoffen.
- ↑ "Misuse of Drugs Act 1971". https://www.legislation.gov.uk/ukpga/1971/38/schedule/2.
 |

