(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description
Dichloropane Clinical data ATC code Legal status Legal status
Identifiers
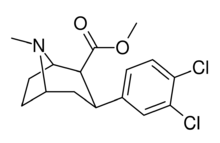
Methyl (1R ,2S ,3S ,5S )-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane-2-carboxylate
CAS Number PubChem CID ChemSpider UNII Chemical and physical data Formula C 15 H 17 Cl 2 N O 2 Molar mass −1 3D model (JSmol )
COC(=O)[C@@H]1[C@H]2CC[C@H](N2C)C[C@@H]1C3=CC(=C(C=C3)Cl)Cl
InChI=1S/C16H19Cl2NO2/c1-19-10-4-6-14(19)15(16(20)21-2)11(8-10)9-3-5-12(17)13(18)7-9/h3,5,7,10-11,14-15H,4,6,8H2,1-2H3/t10-,11+,14+,15-/m0/s1
N Key:JFUNLJPTPCQLIR-PJQZNRQZSA-N
N N Y (what is this?) (verify)
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane , RTI-111 , O-401 ) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 18, and 0.79 nM, respectively.[1] cocaine .[2] [3]
Methylecgonidine is the direct precursor to this compound.[4]
Trans -CO2 Me group The thermodynamic isomer with a trans -CO2 Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials.
See also References
↑ "Synthesis and monoamine transporter binding properties of 3beta-(3',4'-disubstituted phenyl)tropane-2beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry 48 (8): 2767–71. April 2005. doi :10.1021/jm040185a . PMID 15828814 . ↑ "Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine". Psychopharmacology 153 (1): 103–10. December 2000. doi :10.1007/s002130000602 . PMID 11255920 . ↑ "Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat". Psychopharmacology 159 (1): 58–63. December 2001. doi :10.1007/s002130100891 . PMID 11797070 . ↑ "Synthesis, ligand binding, and QSAR (CoMFA and classical) study of 3 beta-(3'-substituted phenyl)-, 3 beta-(4'-substituted phenyl)-, and 3 beta-(3',4'-disubstituted phenyl)tropane-2 beta-carboxylic acid methyl esters". Journal of Medicinal Chemistry 37 (18): 2865–73. September 1994. doi :10.1021/jm00044a007 . PMID 8071935 .
Adamantanes Adenosine antagonists Alkylamines Ampakines Arylcyclohexylamines Benzazepines Cholinergics Convulsants Eugeroics Oxazolines Phenethylamines
1-(4-Methylphenyl)-2-aminobutane 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane 2-Fuoroamphetamine 2-Fuoromethamphetamine 2-OH-PEA 2-Phenyl-3-aminobutane 2,3-MDA 3-Fuoroamphetamine 3-Fluoroethamphetamine 3-Fluoromethcathinone 3-Methoxyamphetamine 3-Methylamphetamine 3,4-DMMC 4-BMC 4-CMC 4-Fluoroamphetamine 4-Fluoromethamphetamine 4-MA 4-Methylbuphedrone 4-Methylcathinone 4-MEAP 4-MMA 4-Methylpentedrone 4-MTA 6-FNE AL-1095 Alfetamine a-Ethylphenethylamine Amfecloral Amfepentorex Amfepramone Amidephrine 2-Amino-1,2-dihydronaphthalene 2-Aminoindane 5-(2-Aminopropyl)indole 2-Aminotetralin Acridorex Amphetamine (Dextroamphetamine , Levoamphetamine )Amphetaminil Arbutamine β-Methylphenethylamine β-Phenylmethamphetamine Benfluorex Benzedrone Benzphetamine BDB BOH 3-Benzhydrylmorpholine BPAP Buphedrone Bupropion Butylone Camfetamine Cathine Cathinone Chlorphentermine Cilobamine Cinnamedrine Clenbuterol Clobenzorex Cloforex Clortermine Cypenamine D -DeprenylDenopamine Dimethoxyamphetamine Dimethylamphetamine Dimethylcathinone Dobutamine DOPA (Dextrodopa , Levodopa )Dopamine Dopexamine Droxidopa EBDB Ephedrine Epinephrine Epinine Etafedrine Ethcathinone Ethylnorepinephrine Ethylone Etilamfetamine Etilefrine Famprofazone Fencamfamin Fencamine Fenethylline Fenfluramine (Dexfenfluramine , Levofenfluramine )Fenproporex Feprosidnine Flephedrone Fludorex Formetorex Furfenorex Gepefrine Hexapradol Hexedrone HMMA Hordenine 4-Hydroxyamphetamine 5-Iodo-2-aminoindane Ibopamine Indanylamphetamine Iofetamine Isoetarine Isoethcathinone Isoprenaline L -DeprenylLefetamine Lisdexamfetamine Lophophine MBDB MDA (tenamfetamine) MDBU MDEA MDMA (midomafetamine) MDMPEA MDOH MDPR MDPEA Mefenorex Mephedrone Mephentermine Metanephrine Metaraminol Mesocarb Methamphetamine (Dextromethamphetamine , Levomethamphetamine )Methoxamine Methoxyphenamine MMA Methcathinone Methedrone Methoxyphenamine Methylenedioxycathinone Methylone Mexedrone MMDA MMDMA MMMA Morforex N,alpha-Diethylphenylethylamine N-Ethylbuphedrone N-Ethylhexedrone N,N-Dimethylphenethylamine Naphthylamphetamine Nisoxetine Norepinephrine Norfenefrine Norfenfluramine Normetanephrine L -NorpseudoephedrineOctopamine (drug) Orciprenaline Ortetamine Oxifentorex Oxilofrine PBA PCA PCMA PHA Pentorex Pentedrone Pentylone Phenatine Phenpromethamine Phentermine Phenylalanine Phenylephrine Phenylpropanolamine Pholedrine PIA PMA PMEA PMMA PPAP Phthalimidopropiophenone Prenylamine Propylamphetamine Pseudoephedrine Ropinirole Salbutamol (Levosalbutamol )Sibutramine Solriamfetol Synephrine Theodrenaline Tiflorex Tranylcypromine Tyramine Tyrosine Xylopropamine Zylofuramine Phenylmorpholines Piperazines Piperidines Pyrrolidines Racetams Tropanes Tryptamines Others
DAT (DRIs
NET (NRIs
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., Desmetramadol|Desmetramadol ]]]], methadone , pethidine (meperidine) , tapentadol , tramadol, Levorphanol
SERT (SRIs
VMATs Others
2-Carboxymethyl Esters (3,4-Disubstituted Phenyl)-tropanes Arylcarboxy Carboxyalkyl Acyl β,α Stereochemistry α,β Stereochemistry Heterocycles: 3-Substituted-isoxazol-5-yl Heterocycles: 3-Substituted-1,2,4-oxadiazole N-alkyl N-replaced (S,O,C) Irreversible Nortropanes (N-demethylated)
Original source: https://en.wikipedia.org/wiki/Dichloropane. Read more